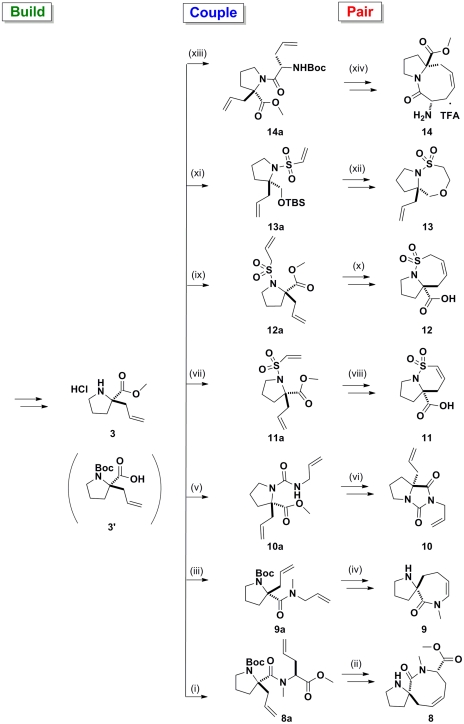
Fig. 3.
Application of a B/C/P approach starting from proline 3/3′ to give compounds 8–14. From 3′, (i) (S)-allylglycine methyl ester, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDCI), Oxyma, Et3N, CH2Cl2, 89%; NaH, MeI, DMF, 89%; (ii) Grubbs II, CH2Cl2, reflux, 34%; TFA; (iii) allylamine, EDCI, Oxyma, Et3N, CH2Cl2, 91%; NaH, MeI, dimethylformamide (DMF), 72%; (iv) Grubbs II, toluene, 60%; TFA. From 3, (v) allyl isocyanate, Et3N, CH2Cl2, 70%; (vi) NaH, DMF, 93%; (vii) 2-chlorosulfonyl chloride, Et3N, CH2Cl2, 62%; (viii) Grubbs II, CH2Cl2, reflux, 92%; LiOH, THF, 53%; (ix) prop-2-ene-1-sulfonyl chloride, Et3N, CH2Cl2, 44%; (x) Grubbs II, CH2Cl2, reflux, 96%; LiOH, THF, 71%; (xi) LiAlH4, THF; tert-butyldimethylsilylchloride, Et3N, CH2Cl2 (24% over two steps); 2-chlorosulfonyl chloride, Et3N, CH2Cl2, 33%; (xii) tetrabutylammonium fluoride, THF, 45%; (xiii) (S)-N-Boc-allylglycine, EDCI, Oxyma, Et3N, CH2Cl2, 48%; (xiv) Grubbs II, CH2Cl2, reflux, 41%; TFA.