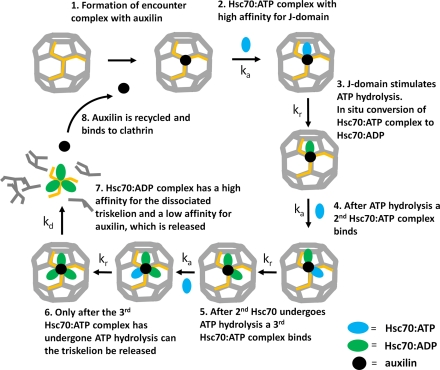
Fig. 5.
An illustration of the serial Hsc70 binding and ATP hydrolysis model for the disassembly of clathrin cages highlighting the sequence of events on a single triskelion. 1: Auxilin has a high affinity for triskelion legs forming part of a clathrin cage and binds at a stoichiometry of one per triskelion. 2: An Hsc70∶ATP complex binds to the clathrin∶auxilin complex. 3: The interaction between auxilin’s J domain and Hsc70 stimulates ATP hydrolysis and induces a conformational change in Hsc70, increasing its affinity for clathrin. 4: Auxilin repositions, and a second Hsc70∶ATP is recruited to the clathrin∶auxilin complex. 5: The second Hsc70 interacts with auxilin’s J domain, ATP is hydrolyzed, and the resulting Hsc70∶ADP complex binds tightly to clathrin. Following hydrolysis, the auxilin again repositions, and a third Hsc70∶ATP binds to the clathrin∶auxilin complex. 6: Hydrolysis of the third ATP results in a weak interaction between the triskelion(Hsc70∶ADP)3 complex and the cage. The rate-limiting step is the disassembly of triskelia leading to cage collapse. 7: The Hsc70∶ADP complex has a high affinity for the released triskelia and remains bound, whereas the affinity of triskelia(Hsc70∶ADP)3 for auxilin is low; the previously bound auxilin is free now to rebind the cage in a catalytic manner (8).