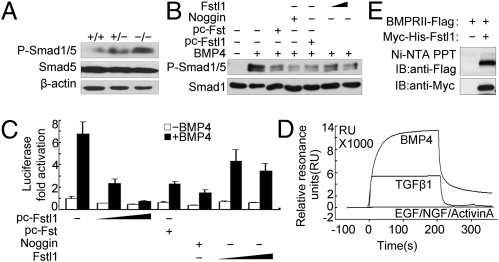
Fig. 5.
Fstl1 modulated BMP4/Smad1/5/8 signaling via binding to BMP4. (A) Phosphorylated Smad1/5 in lung tissues from WT and Fstl1−/− embryos at E18.5. (B) Fstl1 inhibited BMP4-induced phosphorylation of Smad1/5 in Hep3B cells. Recombinant Fstl1 protein (25, 100 ng/mL) or transient overexpression of Fstl1 (pc-Fstl1, 1 μg) inhibited Smad1/5 phosphorylation induced by BMP4 (20 ng/mL). Noggin (25 ng/mL) and pc-Follistatin (Fst) (1 μg) were used as positive controls because they are known BMP antagonists. After 1 h of BMP4 treatment, cells were harvested for immunoblotting. (C) Fstl1 inhibited BMP4-induced expression of the reporter BRE-luciferase activities in Hep3B cells. The construct of BRE-luciferase reporter (0.3 μg) was cotransfected with pc-Fstl1 (0.1 μg, 0.2 μg) or treated with recombinant Fstl1 protein (25 ng/mL or 100 ng/mL). After 16 h of BMP4 treatment, cells were harvested for luciferase assay. Pc-Fst (0.1 μg) or Noggin (25 ng/mL) were used as control. The data represent the mean ± SEM of three independent experiments after normalized to Renilla activity. (D) Sensorgrams of SPR analyses show the binding of Fstl1 to BMP4 or to TGF-β1, not to activin A; EGF and NGF were used as negative controls. (E) Fstl1 binds to type II receptor of BMP4 using a pull-down assay. The Ni-Fstl1-BMPRII protein complex was immunoblotted with anti-Flag antibody to confirm the presence of BMPRII (Upper). Each blot was further developed with anti-Myc antibody to confirm the presence of Fstl1 (Lower).