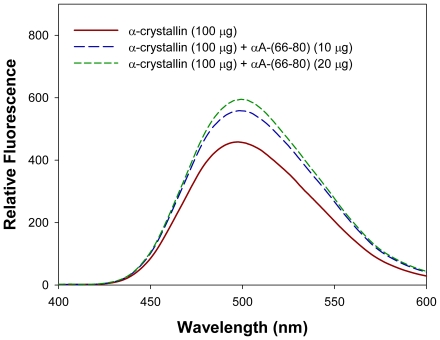
Figure 3. Bis-ANS binding to α-crystallin treated with or without αA-(66-80).
α-Crystallin (100 µg) purified from a bovine lens extract was incubated with and without 10 or 20 µg of αA-(66-80) peptide in 50 mM phosphate buffer, pH 7.2 for 60 min and 10 µL of 20 mM bis-ANS prepared in 5% ethanol was added. Fluorescence was recorded after excitation at 390 nm in a Jasco FP750 spectrofluorometer. The fluorescence for αA-(66-80) was subtracted from the spectra for the complexes. These data show that the interaction of this peptide with α-crystallin leads to an overall increase in the hydrophobicity of the complex.