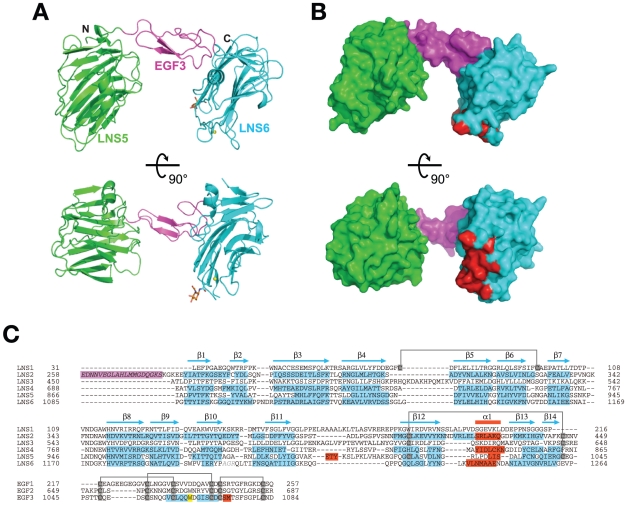
Figure 2. Structure of NX1α(III).
(A) Two different views of the NX1α(III) structure in ribbon presentation, with the LNS5 domain colored in green, the EGF domain in magenta, and the LNS6 domain in cyan. A Ca2+ and an N-acetylglucosamine (GlcNac) residue attached to Asn 1186 are shown as a yellow sphere and a stick model, respectively. (B) Molecular surface of NX1α(III) colored and viewed as in (A). Residues that constitute the NL1-binding site in Nrx1β are colored in red. (C) Multiple-sequence alignment of LNS and EGF domains of Nrx1α. Residue numbers are based on the shortest versions (i.e., without any splice site insertions) of the bovine Nrx1α sequence. For those domains that have structural information, secondary structural elements are highlighted in cyan (β-strands) and red (α-helices), respectively. A segment corresponding to the inserted splicing site 1 (SS1), which was contained in the construct used in this study but which was excluded from the numbering, is highlighted in magenta. Trp1065 in EGF3, which plays an important role in the interdomain interaction, is highlighted in yellow. Conserved disulfide bonds are indicated by gray lines.