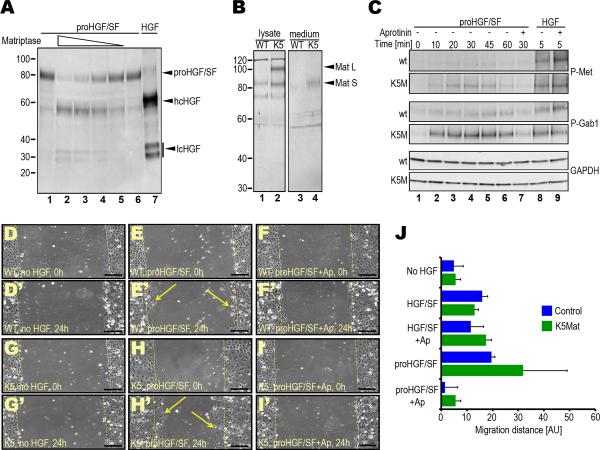
Figure 3. Genetic elevation of matriptase expression potentiates proHGF/SF signaling in primary keratinocytes.
A. Efficient activation of recombinant proHGF/SF by the activated matriptase serine protease domain in solution. proHGF/SF (40 nM) was incubated with 4 (lane 2), 2 (lane 3), 1 (lane 4), and 0.5 (lane 5) nM matriptase or vehicle (lanes 1 and 6) for 1 h at 37 °C. Lanes 1 and 7 are single-chain proHGF/SF and two-chain HGF standards, respectively. Positions of single-chain proHGF/SF, heavy (hcHGF) and light (lcHGF) chains of two-chain HGF/SF are indicated right. Molecular weight markers (kDa) are indicated left. B. Elevated matriptase expression in cultured primary K5-matriptase transgenic keratinocytes. Cell lysates (lanes 1 and 2) and conditioned medium (lanes 3 and 4) from newborn wildtype (lanes 1 and 3) and littermate K5-matriptase+/0 (lanes 2 and 4) keratinocyte cultures and matriptase expression was analyzed by Western blot. Positions of full-length (Mat L) and SEA domain-processed (Mat S) forms of matriptase are indicated with arrowheads. Positions of molecular weight markers (kDa) are indicated at left. C. Elevated matriptase expression increases c-Met and Gab1 phosphorylation in response to single-chain proHGF/SF, but not to active two-chain HGF/SF. Primary keratinocytes from newborn wildtype (panels 1, 3 and 5 from top) and littermate K5-matriptase+/0 (panels 2, 4, and 6 from top) were treated with either 2.5 nM proHGF/SF (lanes 1–7) or active HGF/SF (lanes 8 and 9) for 0 (lane 1), 5 (lanes 8 and 9), 10 (lane 2), 20 (lane 3), 30 (lanes 4 and 7), 45 (lane 5), and 60 (lane 6) min in the absence (lanes 1–6, and 8) or presence (lanes 7 and 9) of the serine protease inhibitor, aprotinin. Phosphorylated c-Met (panels 1 and 2 from top), phosphorylated Gab1 (panels 3 and 4 from top), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (bottom two panels) were detected by Western blotting. D–J. Elevated matriptase expression amplifies the migratory response of primary keratinocytes to single-chain proHGF/SF, but not to active two-chain HGF/SF. Scrape wounds were generated in confluent monolayers of primary keratinocytes from newborn wildtype (WT) (D–F') and littermate K5-matriptase+/0 (K5) (G–I') mice, and the monolayers were treated with vehicle (D, D', G, and G'), 2.5 nM proHGF/SF (E, E', H, and H') or proHGF/SF with 2 μM aprotinin (Ap) (F, F', I, and I') for 24 h. Dashed yellow lines indicate the margins of denuded area at 0 h. Examples of keratinocytes that have migrated into the denuded area after 24 h are shown in E' and H'. Size bars: 100 μm. J. Quantitation of average migration distance of wildtype (blue bars) and littermate K5-matriptase+/0 keratinocytes (green bars) in response to vehicle, proHGF/SF, proHGF/SF and aprotinin (Ap), active HGF/SF and active HGF/SF with aprotinin. Results are shown as mean migration distance ± standard deviation of the mean.