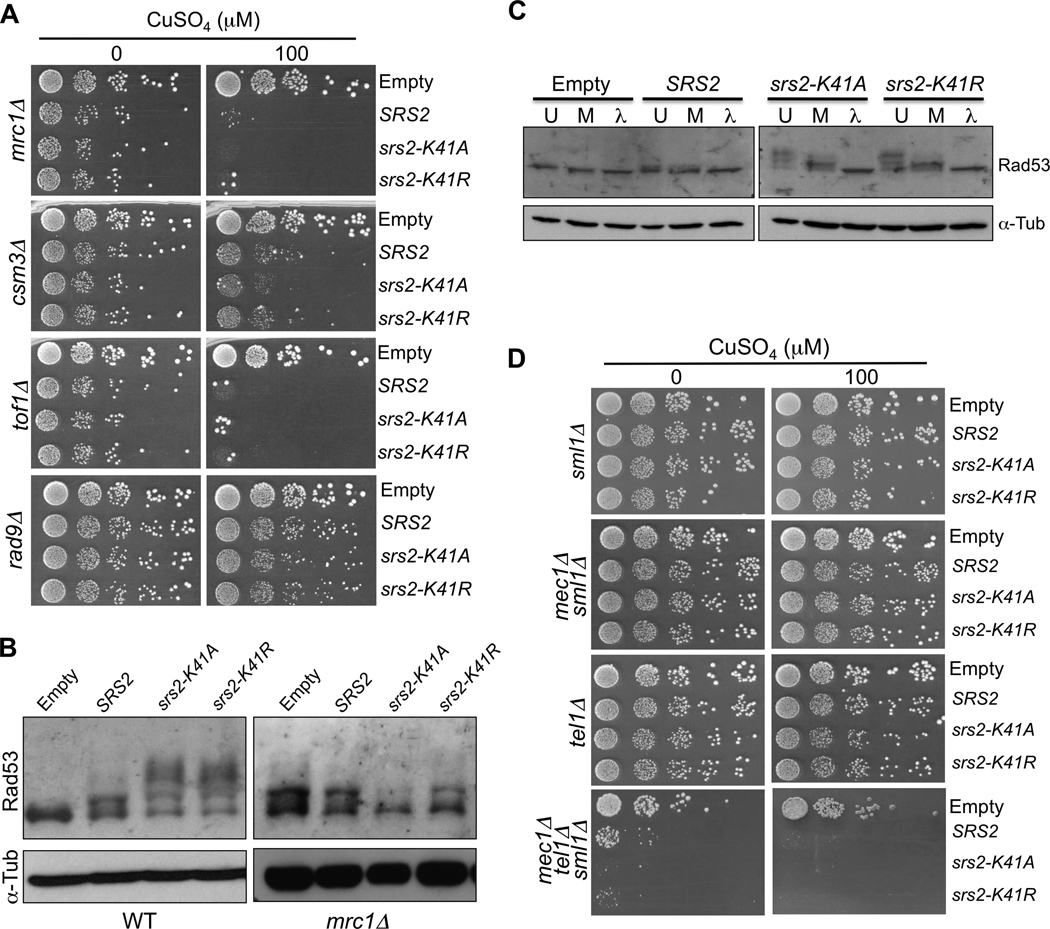
Fig. 2.
Overexpression of wild-type and helicase-dead SRS2 triggers the activation of the replication checkpoint. (A) mrc1Δ, csm3A, tof1Δ or rad9Δ mutants containing the overexpression plasmids were diluted in 10-fold serial dilutions and spotted onto medium without or with copper (100 µM CuSO4). (B) Denaturing protein extracts of wild-type or mrc1Δ cells overexpressing wild-type or mutant SRS2 at 200 µM CuSO4. The proteins were migrated on an acrylamide gel and revealed with antibodies against Rad53 and alpha-Tubulin for a loading control. (C) Rad53 phosphorylation. Native extracts of wild-type cells overexpressing wild-type or mutant SRS2. The extracts were either directly denatured after extraction (U: untreated), or incubated for at 1 hour at 30°C with phosphatase buffer, MnCl2 and protease inhibitors (M: mock), or finally treated with λ-phosphatase for 1 hour at 30°C with phosphatase buffer, MnCl2 and protease inhibitors (λ: λ-phosphatase treated). Note that the Rad53 is partially unphosphorylated in “Mock” samples suggesting the presence of phosphatases in the cellular extracts. (D) sml1Δ, mec1Δ sml1Δ, tel1Δ or mec1Δ tel1Δ sml1Δ mutants containing the overexpression plasmids were diluted in 10-fold serial dilutions and spotted onto medium without or with copper (100 µM CuSO4).