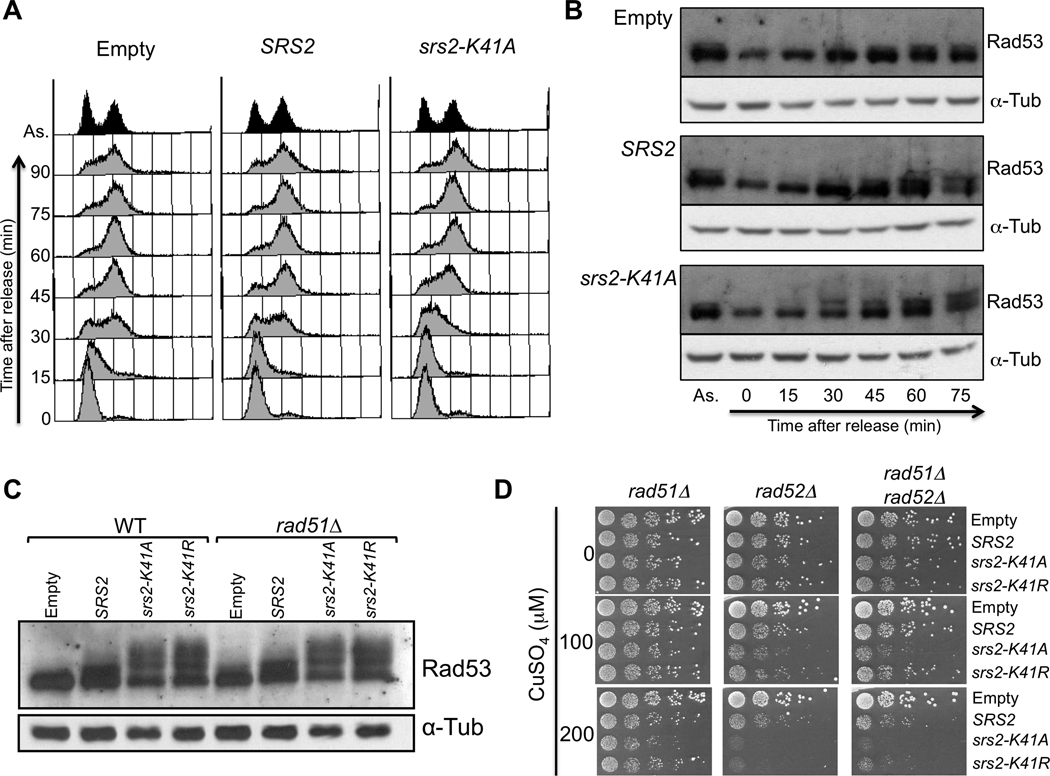
Fig. 3.
Overexpression of wild-type or helicase-mutant SRS2 delays S-phase progression and acts independently of RAD51. (A) MATa bar1Δ cells containing an empty vector control or the plasmids for overexpression of SRS2 or srs2-K41A were synchronized in G1 with alpha factor. To induce overexpression, 200 µM CuSO4 were added 30 minutes prior to release, and the induction was kept upon release. Samples were collected every 15 minutes for FACS analysis. (B) Cells were synchronized as described in (A) and samples were collected for protein extractions. The proteins were migrated on an acrylamide gel and revealed with antibodies againt Rad53 and alpha-Tubulin for a loading control. (C) Denaturing protein extracts of wild-type or rad51Δ cells overexpressing wild-type or helicase-dead SRS2. The extracts were revealed with antibodies against Rad53 and alpha-tubulin as a loading control. (B) rad51Δ, rad52Δ or rad51Δ rad52Δ mutants containing the overexpression plasmids were diluted in 10-fold serial dilutions and spotted onto medium without or with copper (100 µM and 200 µM CuSO4).