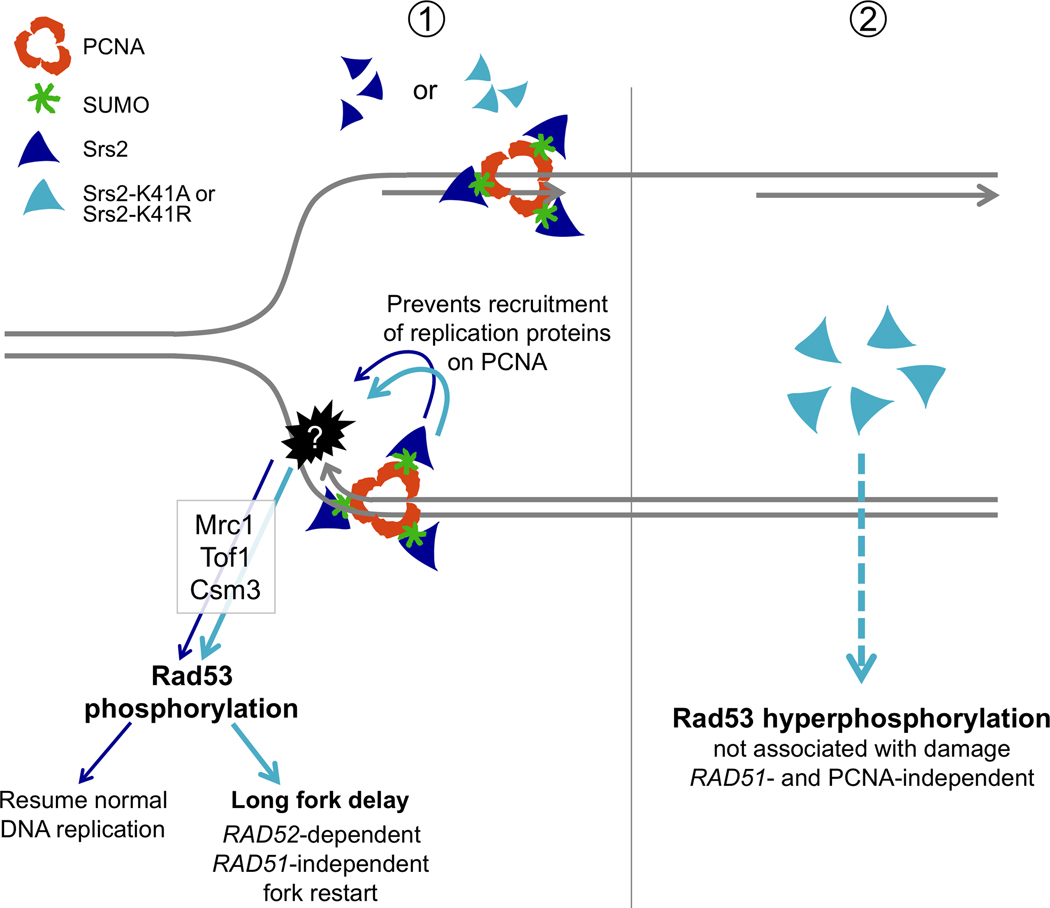
Fig. 6.
Model representing the effects of overexpression of SRS2 and its helicase-dead mutants on DNA replication. ① Wild-type or helicase-dead SRS2 are recruited to replication forks by SUMOylated PCNA. This interaction likely prevents the recruitment of other factors required for replication, leading to fork progression delay, which activates the replication checkpoint (Mrc1, Tof1 and Csm3-mediated phosphorylation of Rad53). Cells overexpressing SRS2 eventually overcome this defect. The helicase-dead mutants srs2-K41A or srs2-K41R show a greater replication delay that likely leads to the accumulation of aberrant intermediates that require RAD52-dependent, RAD51-independent recombination to resume replication. ②The helicase-dead mutants induce activation of the DNA integrity checkpoint, resulting in hyperphosphorylation of Rad53. This effect is independent of DNA replication, is not associated with any damage and likely results from the accumulation of an Srs2 protein capable of triggering checkpoint activation.