Summary
Studying saccades can illuminate the more complex decision-making processes required for everyday movements. The double-step task, in which a target jumps to two successive locations before the subject has time to react, has proven a powerful research tool to investigate the brain’s ability to program sequential responses. We asked how patients with a range of cerebellar disorders responded to the double-step task, specifically, whether the initial saccadic response made to a target is affected by the appearance of a second target jump. We also sought to determine whether cerebellar patients were able to make corrective saccades towards the remembered second target location, if it were turned off soon after presentation. We tested saccades to randomly interleaved single- and double-step target jumps to eight locations on a circle. Patient’s initial responses to double-step stimuli showed 50% more error than saccades to single target jumps, and often, they failed to make a saccade to the first target jump. The presence of a second target jump had similar, but smaller effects in control subjects (error increased by 18%). During memory-guided double-step trials, both patients and controls made corrective saccades in darkness to the remembered location of the second jump. We conclude that in cerebellar patients, the second target jump interferes with programming of the saccade to the first target jump of a double-step stimulus; this defect highlights patients’ impaired ability to respond appropriately to sudden, conflicting changes in their environment. Conversely, since cerebellar patients can make corrective memory-guided saccades in darkness, they retain the ability to remember spatial locations, possibly due to non-retinal neural signals (corollary discharge) from cerebral hemispheric areas concerned with spatial localization.
Keywords: Saccades; double-step; dysmetria; cerebellum, fastigial nucleus; efference copy
Introduction
In everyday life, as we move through our environment, we are required to change our behavior in response to obstacles in the terrain and moving objects. For example, a visual target may jump from one point to another requiring a sequence of movements of eyes, limbs, and gait. Normal subjects can take such environmental perturbations in their stride and even plan their movements based on memories of the locations of moving objects. Such behavior has been studied in a reductionist way by measuring responses of rapid eye movements (saccades) to double-step stimuli, in which the visual target jumps successively to two locations even before an eye movement can be generated (Westheimer, 1954;Young & Stark, 1963;Becker & Jurgens, 1979;Camalier et al., 2007). This strategy has provided insights into the contributions of a number of circuits involved with the generation of sequences of saccades, from brainstem (Goossens & Van Opstal, 1997) to basal ganglia (Joti et al., 2007;Farooqui et al., 2010), and cerebral cortex (Duhamel et al., 1992;Heide et al., 1995;Sommer & Wurtz, 2002).
The ability to successfully perform a sequence of behaviors depends heavily upon the cerebellum, which plays an important role in “motor learning” including adaptations necessary to hone performance of skills as varied as piano playing or dancing (Iwamoto & Kaku, 2010). Analyzing saccades provides several advantages for studying motor adaptations and has demonstrated, for example, that patients with cerebellar disease have impaired ability to adjust saccades to meet new visual demands (Alahyane et al., 2008;Iwamoto & Kaku, 2010). The limited ability of cerebellar patients to “change their mind” is also evident, for example if they are required to turn suddenly in order to avoid collision, they are prone to fall. Neurologists are aware of this defect and routinely safeguard cerebellar patients against falls as they test their ability to change course during gait testing (Manto, 2010). Accordingly, we set out to determine whether patients with cerebellar disease showed more errors on the saccade double-step test than did control subjects. We looked at two specific aspects of this test: (1) whether the presence of a second target jump affected the accuracy of initial saccades compared with responses to single target jumps; and (2) whether subjects could make accurate memory-guided saccades toward the second target location, if it were turned off soon after the jump. To our knowledge, the first question has not been previously addressed and prior studies have been inconclusive on the latter point (Kanayama et al., 1994;Gaymard et al., 1994;Bronstein et al., 1995). A variant of the double-step paradigm, in which the target predictably jumps back as the saccade is made, has been used extensively to study the cerebellar role in motor learning (McLaughlin, 1967;Takagi et al., 1998;Kojima et al., 2010). However, in our study, we minimized adaptive behavior by presenting single- and double-step stimulus jumps to eight possible target locations in a non-predictable sequence. We found that the predominant deficit in cerebellar disease concerned programming of saccades to the first target jump of a double-step. Conversely, the ability to direct saccades to the remembered location of a target was retained in patients with cerebellar disease. Preliminary results have appeared as an abstract (King et al., 2010).
Methods
Subjects
We examined 10 patients with evidence of cerebellar disease (8 male and 2 female; age range 31-85 years, median= 59); diagnoses, medications, and other characteristics are summarized in Table 1. All cerebellar patients underwent a formal neurological evaluation prior to selection; only those patients with normal mental status who clearly understood the nature of the testing were included. In view of the physical disabilities that afflicted most of our patients, we were only able to conduct single test sessions lasting about 30 minutes. We also tested 10 age- and gender-matched healthy control subjects (age range 26-70 years, median=52). All individuals gave signed, informed consent in accordance with our Institutional Review Board and the Declaration of Helsinki. Testing took place in a dark room, and an investigator remained in the room and encouraged all subjects during the session.
Table 1. Summary of Clinical Information on Patients Studied and pooled Normal Subjects (first row).
Disturbances of eye movements are listed in order of prominence on examination. aVOR: angular vestibulo-ocular reflex. DBN: downbeat nystagmus. EA2: episodic ataxia type 2. GEN: gaze-evoked nystagmus. MRI: magnetic resonance imaging of head; MSO: macrosaccadic oscillations. SCA3-MJD: spinocerebellar ataxia type 3 – Machado-Joseph disease. SCA6: spinocerebellar ataxia type 6. SCASI: spinocerebellar ataxia with saccadic intrusions. UBN: upbeat nystagmus.
| Subject/Age/ Sex |
Diagnosis/ Duration |
Neurological & Neuro- imaging Findings |
Disturbance of Eye Movements |
9-Hole Peg Test |
Medicines |
|---|---|---|---|---|---|
| NS/52*/2F & 8M |
Controls | Normal | Normal | R: 18 s** L: 21 s** |
NA |
| CP1/58/M | SCASI/30 | Limb, trunk, & gait ataxia, dysarthria, neuropathy. MRI: diffuse cerebellar atrophy |
Saccadic hypermetria, mainly horizontal; MSO |
R: 44 s L: 44 s |
Memantine Valproate Pramipexole Gabapenti |
| CP2/62/M | SCASI/32 | Limb, trunk, & gait ataxia, dysarthria, neuropathy. MRI: diffuse cerebellar atrophy |
Saccadic hypermetria, mainly horizontal; MSO |
R: 45 s L: 48 s |
Memantine Valproate Clonazepam Fluoxetine |
| CP3/56/M | SCA3-MJD/35 | Limb & gait ataxia, dysarthria. MRI: cerebellar atrophy, most marked in vermis |
Saccades dysmetric & mildly slowed, vertical > horizontal; GEN; aVOR impaired |
R: 54 s L: 52 s |
Metoprolol |
| CP4/39/M | EA2/30 | Limb & gait ataxia during attacks, dysarthria. MRI: diffuse cerebellar atrophy |
GEN with downbeating component; saccadic dysmetria; impaired pursuit |
R: 25 s L: 20 s |
Acetazolamide Quetiapine Lamotrigine |
| CP5/59/F | SCA6/6 | Gait ataxia, photophobia. MRI: diffuse cerebellar atrophy |
GEN with downbeating component; impaired vertical aVOR and pursuit; saccadic dysmetria |
R: 25 s L: 22 s |
Acetazolamide Bupropion |
| CP6/78/M | Right vestibular schwannoma, 3 cm × 6 cm × 3 cm /1.5 resected 21 months previously |
Right-sided deafness, dysarthria, & limb ataxia; gait ataxia, vertical diplopia. MRI: Residual right-sided tumor with compression of middle cerebellar peduncle & hemisphere |
GEN on right gaze with upbeat component; impaired smooth pursuit and aVOR; left hypertropia; saccadic dysmetria; |
R: 34 s L: 27 s |
Prazosin |
| CP7/85/M | Sporadic cerebellar degeneration/7 |
lower limb & gait ataxia, mild diabetic neuropathy. MRI: normal for age |
DBN increasing on lateral gaze, with GEN; skew deviation with left hypertropia; mild saccadic hypometria; impaired vertical pursuit |
R: 29 s L: 27 s |
Metoprolol |
| CP8/67/M | Sporadic cerebellar degeneration/7 |
Gait ataxia, dysarthria, mild memory deficit with emotional swings. MRI: normal for age |
DBN increasing on lateral and down gaze and at near- viewing; abnormal “cross- axis” upward eye movements |
R: 31 s L: 26 s |
Buspirone |
| CP9/33/F | Wernicke- Korsakoff syndrome/1.5 |
Limb & gait dysmetria, mild short-term memory deficits. MRI: normal |
UBN switching to DBN at near and in right lateral gaze; aVOR impaired |
R: 23 s L: 18 s |
Lorazepam Gabapentin Topiramate Sertraline Clonazepam |
| CP10/60/M | Idiopathic downbeat nystagmus/2 |
Vertical oscillopsia, mild gait imbalance. MRI: normal |
Vertical diplopia due to DBN (worse during right gaze) |
R:19 s L: 19 s |
none |
Subject ages and disease duration are in years.
median age of normal subjects.
median of 9-Hole Peg Test results for normal subjects.
Eye movement measurements
We measured horizontal and vertical eye movements as subjects sat in magnetic fields with a scleral search coil on one eye, after application of topical anesthetic (Robinson, 1963). The coil system was pre-calibrated by moving a centrally mounted protractor to specific gaze angles and adjusting corresponding offsets and gains of voltages. During testing, subjects were seated in a stationary chair with a headrest to restrain movement.
Experimental stimuli and paradigms
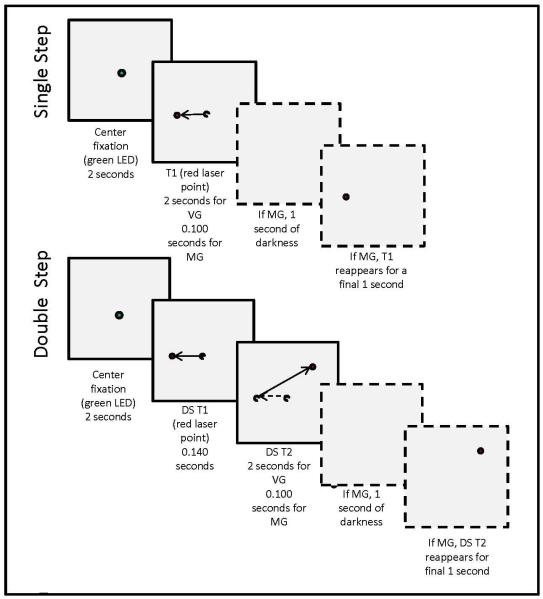
Subjects viewed targets projected onto a tangent screen at a viewing distance of 1.2 m. We tested saccades to 208 randomly interleaved visually-guided and memory-guided single-step (SS, 60% of stimuli) and double-step (DS, 40% of stimuli) target jumps (Figure 1). Each trial started as subjects viewed a central green target. After 2 s, the fixation target went out and a red target (T1) immediately appeared at one of 8 locations on the circumference of a circle with a 10 degree radius. The position of the projected red laser-diode target was controlled by an X-Y mirror galvanometer (General Scanning DX 2003), with mean latency of 6 ms, under computer control; appearance and disappearance of visual stimuli were determined by turning the laser diode on and off (latency < 2 ms), so the target jumped crisply from one location to another. For SS trials, T1 remained on for 2 s, but for DS trials T1 was present for only 140 ms and a second target (T2) appeared in one of 5 possible locations with a minimum 90 deg directional separation from T1. We selected a T1 duration of 140 ms based on a prior study which successfully examined use of efference copy in a double-step task (Heide et al., 1995). In memory-guided trials, the final target (T1 for SS, T2 for DS) was present for 100 ms followed by 1 s of darkness before reappearing for a final 1 s. Every testing session began with a demonstration of each type of stimulus, providing the subject with adequate time to understand the tasks. Subjects were instructed to look at the targets as they appeared. We did not ask them to cancel the initial saccade to the first target of double-step stimuli (Ray et al., 2004), since we sought to promote a mental state similar to that during normal activities. In the case of memory-guided trials, we asked subjects to look at the remembered location of the final target after it disappeared.
Figure 1.
Examples of possible visual stimuli for single and double-step paradigms with timing indicated. Boxes with solid outlines illustrate sequences for visually-guided (VG) stimuli while boxes with dotted outlines show the additional steps involved in memory-guided (MG) stimuli. Visual targets (colored circles) were presented at one of 8 locations on the circumference of a circle with a 10 degree radius; the fixation target lay at the center of this circle (see Methods for details). Dotted circles and arrows represent previous target locations and normal eye movements.
Data analysis
Search coil signals (voltages) were filtered (bandwidth 0-150 Hz) before digitization at 500 Hz. Experimental stimuli and data acquisition were programmed using a LabVIEW computer interface (National Instruments, Austin, TX); analysis programs were written using MatLab (MathWorks, Natick, MA). Eye velocity and acceleration were calculated from eye position as previously described (Ramat et al., 1999). Saccades were defined as target-directed eye movements faster than 10 deg/s; their onset and offset were determined by when the velocity rose above or fell below a 10 deg/s threshold, respectively. Records were analyzed interactively, and data contaminated by blinks or non-saccadic eye movements were discarded.
We sorted saccadic responses into visually-guided and memory-guided categories each with single-step (SS), first target of double-step (DS1), and second target of double-step (DS2) trial types for comparison. When subjects did not make a saccadic response to DS1, we noted this as a “missed” trial. Also, a trial was coded as “missed” if the direction of the initial saccade was greater than 45° away from DS1. For each individual subject, amplitudes were calculated for initial eye displacement (eye position after initial saccade to target – starting eye position), final eye displacement (eye position after final saccade to target – starting eye position), and target displacement (target jump position – starting target position). Responses were normalized to account for small individual differences in the distance between the subject’s eye and the tangent screen, using the median ratio of final eye displacement /target displacement for SS stimuli to scale the eye coil signal for all responses.
We computed the horizontal and vertical components of the absolute value of saccadic error (target position – eye position) for the initial and final movements to single-steps and both components of double-steps. In the case of memory-guided saccades, we measured the error of initial eye position in darkness and compared this with the error of final eye position in darkness before the target reappeared in order to determine if subsequent saccades made to memorized target positions were corrective. We chose to analyze horizontal and vertical errors separately since cerebellar patients often show a specific pattern of dysmetria, which is idiosyncratic but consistent for each patient. For example, hypermetria may be prominent horizontally but less evident vertically. Although we also measured saccadic gain (amplitude of initial or final eye displacement /amplitude of target displacement), we found error measurements more reliable (Kori et al., 1998) and present these data here. We also measured the latency to onset of each saccadic response (time of saccade onset – time of stimulus onset).
Our primary analysis was between pooled data from the groups of cerebellar patients and normal subjects; for this we used the Mann-Whitney Rank Sum Test since data were not normal in distribution. We also compared each cerebellar patient’s data set against the corresponding pooled data from control subjects using Kruskal-Wallis One-way ANOVA on Ranks with ten degrees of freedom and used Dunn’s Method for multiple comparisons versus a control group. Unless otherwise specified, statistical significance corresponded to p < 0.05.
Results
Accuracy of Visually-Guided Saccades
As expected, cerebellar patients (CP) were less accurate than normal subjects (NS) when they made saccades to single-step target jumps (SS). A representative plot is shown in Figure 2A and data from all patients are summarized in Figure 3 (compare NS/SS and CP/SS). All differences between cerebellar patients and normal subjects for corresponding test conditions were significant (p<0.001). Furthermore, patients’ saccades demonstrated greater variance of error than did those of normal subjects, with these differences more apparent for the horizontal (145% greater) than vertical components (31% greater).
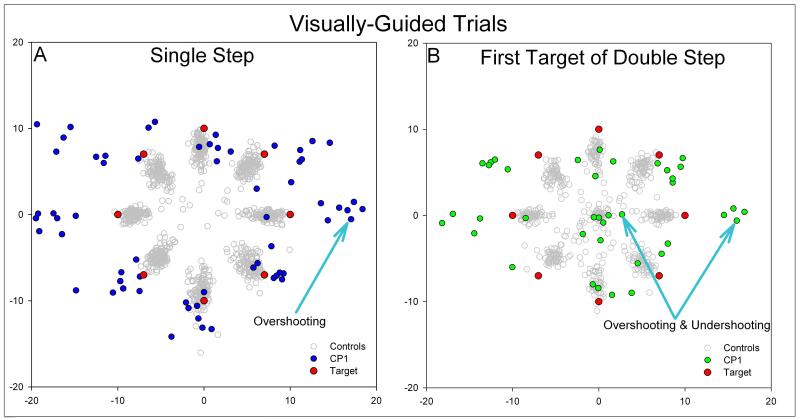
Figure 2.
Representative plots of eye position after the initial saccadic response to single-step stimuli (A) and the first target jump of double-step stimuli (B). Blue or green circles are endpoints of saccades made by CP1, and red circles denote the 8 possible laser target positions. Gray circles are pooled responses from control subjects. In response to single step stimuli (A), CP1 showed hypermetria, predominantly in the horizontal direction. However, in response to the first target jump of double-step stimuli (B), CP1 made both hypermetric and hypometric saccades in the horizontal direction. This variation in accuracy was found for initial saccades made to double-step stimuli in which the second target jumped into either the same or opposite hemifield. CP1’s responses differed from normal subjects whose initial saccades (gray circles) are either on target or hypometric for both single step and double step stimuli.
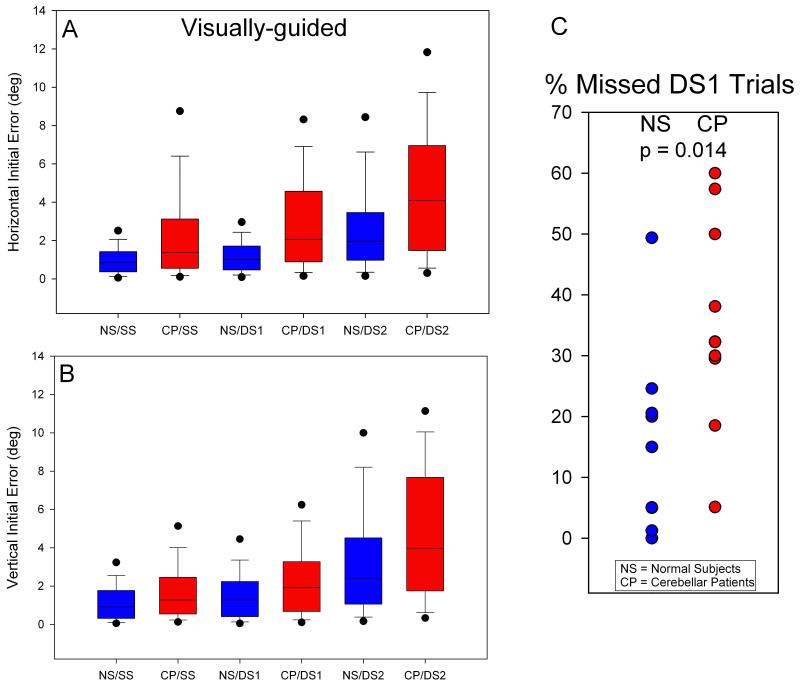
Figure 3.
Summary of horizontal (A) and vertical (B) errors for visually guided saccades and percentage of trials with no response to the first target jump of double step (C). Errors of normal subjects are displayed as blue box-plots with percentile values while those of cerebellar patients are in red. For all step types, patients are more inaccurate (larger median error and variance) than controls, horizontally and vertically. But, differences between patients and controls are greater for horizontal components of initial saccades. Further, the difference between CP/SS and CP/DS1 is greater than between NS/SS and NS/DS1 (A & B). Cerebellar patients failed to make any saccade to the first target of a double step about twice as often as controls as shown in (C).
Cerebellar patients also performed inferiorly to normal subjects for the initial saccade made to the first target jump of a double-step stimulus (DS1). In fact, patients failed to make any saccade at all to DS1 twice as often as normal subjects (Figure 3C). When they did generate a saccade in response to DS1, patients showed greater errors and variance than normal subjects (Figure 3A and B, compare NS/DS1 and CP/DS1; p<0.001); these differences were greater for horizontal than vertical components (32% greater error & 110% greater variance). A representative example is shown in Figure 2; note how the pattern of dysmetria switches from consistent overshoots in Figure 2A (SS) to undershoots and overshoots in Figure 2B (DS1).
We were especially interested in determining whether the presence of a second target step affected the accuracy of the response to the first target jump (DS1). When we compared the error of initial saccades to SS versus DS1, normal subjects showed 18% increase in error, but patients showed 50% increase (Figure 3A; compare NS/SS with NS/DS1 and CP/SS with CP/DS1).
Accuracy of Memory-Guided Saccades
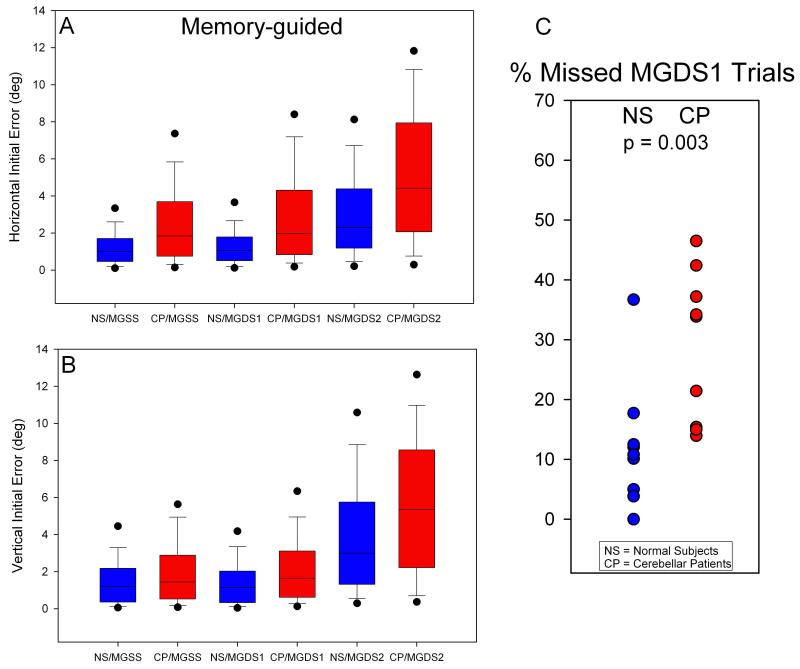
Similar to visually-guided trials, cerebellar patients also made less accurate saccades than did normal subjects to memorized SS (MGSS) and to DS1 when DS2 was memorized (MGDS1 & MGDS2); see (Figure 4A-B, p<0.001). Differences between the accuracy of saccades to SS and DS1 were larger when the final targets were visible than when they were remembered, for both CP and NS. However, cerebellar patients still failed to generate a saccade to MGDS1 significantly more often than did normal subjects (Figure 4C).
Figure 4.
Summary of errors for memory guided saccades. Conventions are similar to Figure 3. Differences between controls and patients and between responses to single steps and response to the first step of a double-step stimulus are less marked but similar to those found for visually guided saccades.
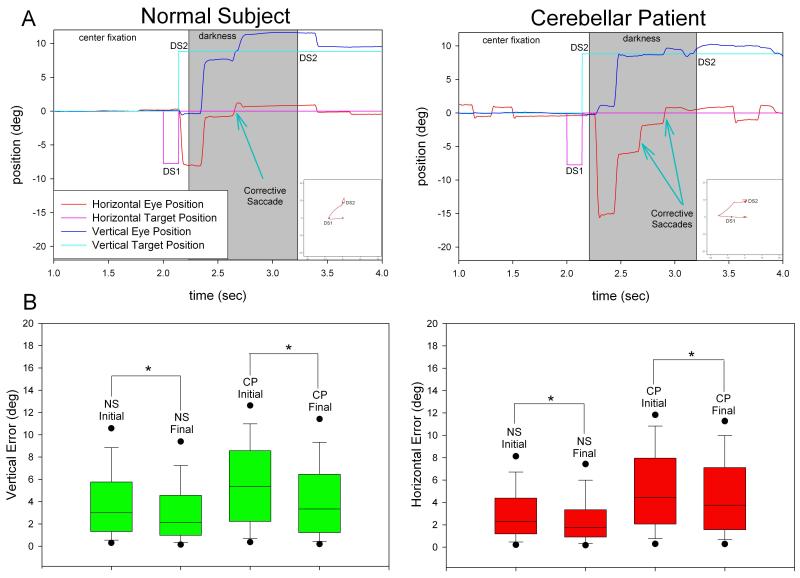
In the case of memory-guided saccades, we aimed to resolve whether cerebellar patients are able to make corrective saccades to remembered target positions in darkness. We found that, similar to normal subjects, patients did make corrective saccades in darkness to memorized DS2, with final position error being statistically smaller than initial position error (p<0.001). A representative record is shown in Figure 5A and data from all subjects are summarized in 5B.
Figure 5.
(A) Representative raw data plots of a normal subject (left) and a cerebellar patient (right), with predominant horizontal dysmetria, showing that both make corrective saccades to the memorized second target of double step. (B) Summary of errors for saccades made to the memorized second target of double step for horizontal (left) and vertical (right) components in normal subjects and cerebellar patients. The final saccadic error, for both normal subjects and patients, is less than the initial saccadic error during darkness; this demonstrates that saccades subsequent to the initial movement are made towards the memorized target location and are truly corrective.
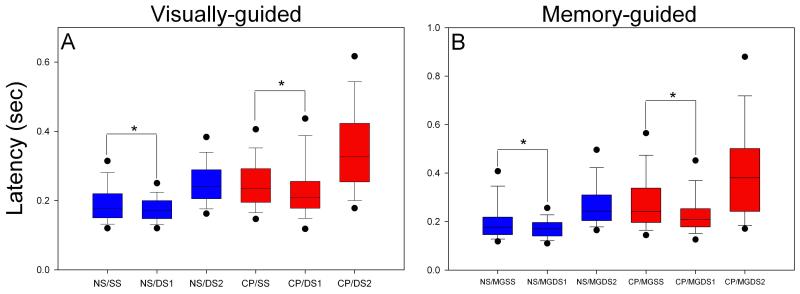
Latency of Saccades
Cerebellar patients showed longer latency to onset of saccades on all trial types, both horizontally and vertically (Figure 6; p<0.001). Saccadic latency for the first target of a double step (DS1 & MGDS1) was statistically shorter (p<0.001) than for a single step target (SS & MGSS); this finding was greater in cerebellar patients than in normal subjects. When we plotted the intervals between saccades made to DS1 and DS2 (intersaccadic interval) as a function of the delay of the first saccade to DS1 (delay time = latency of saccade to DS1 – 0.140 s), we found a negative slope for both cerebellar patients (−0.28) and normal subjects (−0.40) which is evidence for parallel processing (Becker & Jurgens, 1979). If parallel processing is occurring, then a longer delay time should allow more time for processing the response to DS2, so that the intersaccadic interval becomes shorter. In order to determine if the longer latencies of patients contributed to increased error of initial saccades made to DS1, we performed a Spearman correlation test. We found no correlation (P>0.05) between latency and error in visually- and memory-guided trials for either patients or normal subjects. Furthermore, saccades made by normal subjects with longer latencies, which overlapped with latency values of patients, were not associated with decreased accuracy.
Figure 6.
Summary of latencies for visually-guided (A) and memory-guided (B) saccades made by normal subjects (NS) and cerebellar patients (CP). Both normal subjects and patients demonstrate shorter latencies for saccades made to DS1 than made to SS target jumps; this is also true for memory-guided saccades.
Secondary Analysis of Differences between Individual Patients
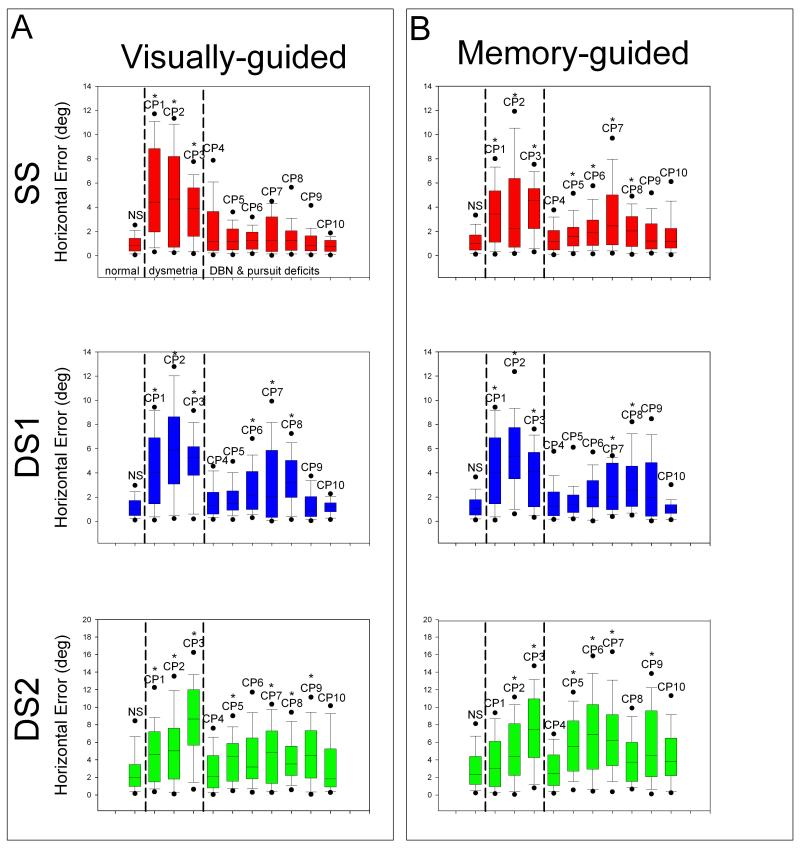
Each cerebellar patient showed at least one abnormality of saccade accuracy or latency. However, since the patients represented a variety of cerebellar disorders, we investigated whether the findings summarized above were more prominent in certain patients. We were especially interested in differences between those patients showing predominant saccadic dysmetria (CP1-3, associated with disease affecting the dorsal vermis and fastigial nucleus) versus those with gaze-holding and pursuit deficits (CP4-10, associated with disease affecting the vestibular cerebellum) – see Figure 7.
Figure 7.
Comparison of initial horizontal saccade error between normal subjects (first boxplot of each graph) and individual cerebellar patients (CP1-10). Normal subjects, those patients showing predominant dysmetria, and patients showing predominant DBN & pursuit deficits are indicated in the top left panel and apply to all graphs. (A) Visually-guided saccade data for SS (red), DS1 (blue), and DS2 (green). Note that CP1-3 in particular show greater error than normal subjects. Also, majority of cerebellar patients have increased variance of error compared with normal subjects. Furthermore, patients are more inaccurate (greater median error and variance) for DS1 than SS target jumps. (B) For saccadic error in memory-guided trials, trends are similar to those found in visually-guided saccades.
For visually-guided single-step target jumps, patients with predominant saccadic dysmetria (CP1-3) showed significantly greater error for horizontal components of saccades than did controls, while patients with gaze-holding deficits (CP7-8) mostly demonstrated greater error for vertical components of saccades. Variance in error for horizontal components of saccades was greater for both groups of patients compared with controls. For vertical components, none of the patients’ variance differed significantly from controls with the exception of CP3 who showed a 136% increase in the variance of saccade error.
The presence of a second visually-guided target jump caused more individual patients to be less accurate than normal subjects, horizontally and vertically (6 patients indicated by asterisk for DS1 (blue) versus 3 asterisked patients for SS (red) in box plots in Figure 7A). Patients with marked saccadic dysmetria (CP1-3) demonstrated greater error than did controls for horizontal components of initial saccades made to DS1, but so did most patients with gaze-holding and smooth pursuit deficits (CP6-8; p<0.05). For both groups of patients, accuracy decreased for vertical components of initial saccades made to DS1 compared with normal subjects (CP3 & CP8; p<0.05), although to a lesser extent than for horizontal components. Variance in error for saccades made to DS1 increased for both groups of individual patients compared with normal subjects, but only significantly for horizontal components of saccades (CP1-3 up to 333% & CP6-8 up to 325%).
For memory-guided single-step target jumps, compared with visually-guided jumps, patients with predominant saccadic dysmetria and most patients with gaze-holding and pursuit deficits showed greater inaccuracy and variance of error than did controls for horizontal components of saccades (CP1-3, CP5-8; p<0.05). Patients with gaze-holding deficits (CP6-8), with the exception of CP3, showed increased error for vertical components of saccades than controls did (p<0.05). An increase in variance of error for vertical components was only noted in two patients, one with saccadic dysmetria (CP3, 159% increase) and one with DBN (CP7, 82% increase) (p<0.05).
For memory-guided double-step target jumps, all patients with predominant saccadic dysmetria (CP1-3) and two patients with DBN (CP7-8) showed greater error than did controls for horizontal components of saccades (p<0.05). However, a more striking finding was that variance in error for horizontal components was increased 227-307% more than controls for all patients with notable saccadic dysmetria and a majority of patients with gaze-holding deficits (In Figure 7 note CP1-3 & CP6-9; p<0.05). Again, patients with gaze-holding and pursuit deficits (CP7-8) made less accurate vertical saccades than controls did, although 1 patient with saccadic dysmetria (CP3) also showed increased error and variance of error for vertical components (p<0.05).
Overall, patients with saccadic dysmetria showed deficits when making eye movements to horizontal target jumps, while patients with gaze-holding and smooth pursuit difficulties demonstrated less accuracy for both horizontal and vertical target jumps. Most importantly, for both groups (i.e., all patients), the presence of a second target decreased accuracy, horizontally and vertically, and significantly increased variation of error. This main finding was consistent and irrespective of other factors such as age and medication.
Discussion
We studied two aspects of saccade control in patients with a range of cerebellar disorders, using the double-step paradigm (Figure 1). First, we asked whether saccades made to the first target jump of the double-step stimulus differed in patients versus controls. A related question was whether the appearance of a second target changed saccadic properties compared with responses to single target jumps. We found that cerebellar patients made less accurate saccades than controls and often did not respond at all to the first of two target jumps (Figure 3). This behavior differed from saccades to single target jumps, which were more accurate for both patients and controls. Second, we asked whether cerebellar disease disrupted the ability to make accurate saccades to the remembered second target position of a double-step stimulus. We found that although our cerebellar patients’ initial saccades were dysmetric, they could make corrective saccades in darkness towards the remembered target location (Figure 5). Each of these findings raises several issues for discussion. First, how do responses by our cerebellar patients relate to prior studies of normal subjects using the double-step task? Second, what insights do our patients’ difficulties in responding to the first target jump when followed by a second target jump provide about the normal governance of saccades by the cerebellum? Third, what is the significance of our finding that cerebellar patients could make memory-guided corrective saccades in regards to the role of non-retinal neural signals (corollary discharge or efference copy) in the programming of eye movements? And finally, what are the clinical implications of these findings?
Saccade Processing and the Double-Step Task
Most saccades are brief; their duration is typically less than the reaction time of the visual system (about 100 ms). Thus, if a visual target jumps to a new location and then back to its original position within 100 ms, subjects respond later by making a saccade to the new location and then back to the origin (Westheimer, 1954). This finding initially led to the conclusion that visual information is processed serially, with a fixed refractory period between one saccade and the next (Young & Stark, 1963). Subsequent studies using double-step stimuli have shown that visual information may be acquired and used to modify a saccade up to 70 ms before it begins (Becker & Jurgens, 1979). Moreover, there is no fixed refractory period (the time used to process the second saccade), so that there may be virtually no intersaccadic interval between responses to closely timed double-step target jumps. With shorter intersaccadic intervals, which require overlap in processing of the two movements, the saccade made to the first target jump becomes increasingly hypometric if the second target jump is oppositely directed (Becker & Jurgens, 1979;McPeek et al., 2000). We found that normal subjects made more hypometric saccades to the initial target jump of a double-step stimulus compared to saccades made to a single target jump (Figure 3). This interference caused by a second target jump was even more marked in our cerebellar patients, who showed both hypermetria and hypometria, and who often failed to make a saccade in response to the first of two target jumps. These results imply that programming a saccade to the second target jump interferes with programming of the saccade to the first target jump. They also indicate that the cerebellum plays a crucial role in governing the accuracy of two, successive motor responses.
Based on studies of saccadic latency to double-step stimuli, it has been proposed that there is a race between “GO1” processing for the saccade to the initial target jump (DS1), “GO2” processing for the saccade to the second target jump (DS2), and “STOP” processing for DS1, of which GO2 and STOP are both triggered by the appearance of the second target (Camalier et al., 2007). Consistent with the basic premise of stochastic independence of the finish times of the two racing processes for the GO1−GO2+STOP race, it has been predicted that DS1 saccade latencies would be either equal to or shorter than latencies to SS target jumps (Camalier et al., 2007). Although our experiment was not designed to test the race model, we found this prediction to be borne out in both normal subjects and cerebellar patients, especially the latter (Figure 3). Another deduction of the study by Camalier and colleagues was “The fact that GO2 and STOP occur at the same time suggests that they may in fact be the same process.” If correct, then for cerebellar patients, it appears that the STOP/GO2 signal disrupts programming of the saccade to the first target jump and may even prevent it from being generated (Figure 3C).
Further insights into parallel processing of saccades come from studies of patients with slow saccades. Such individuals make saccades lasting several hundred milliseconds, making it is possible to jump the visual target to a new location during the course of the movement (Zee et al., 1976;MacAskill et al., 2000). In such cases, the eye turns around in mid-flight and heads to the new target – with no intersaccadic interval during the movement. It appears that omnipause neurons (OPNs) (Sparks, 2002;Horn, 2006;Ramat et al., 2007), which inhibit burst neurons (BNs) except during saccades, remain silent as the eye changes direction without an intersaccadic interval. Thus, these patients show no evidence of an obligatory refractory period, and their saccades are not ballistic, since they can be re-programmed in midflight. How might these findings be related to the cerebellar role in controlling the metrics of saccades?
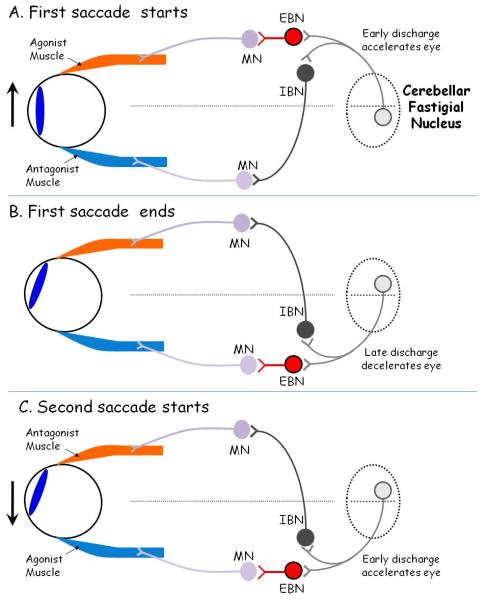
Cerebellar Influences on Saccadic Accuracy (Figure 8)
Figure 8.
Hypothetical scheme to account for the role of the fastigial nucleus of the cerebellum in governing saccades in response to the double-step target paradigm, in which the target (not shown) jumps first to the right and then to the left. EBN: excitatory burst neuron; IBN: inhibitory burst neuron; MN: motoneuron; OPN: omnipause neurons. A: At the onset of a rightward saccade (indicated by upward arrrow), the agonist muscle contracts vigorously due to a pulse of innervation, generated by EBNs, which project monosynaptically to MNs. The antagonist muscle relaxes due to inhibition of its MNs by IBNs. The left cerebellar fastigial nucleus ocular motor region (FOR) promotes the onset of the saccade by its projections to contralateral EBNs and ipsilateral IBNs. B: At the end of the first saccade, the right FOR promotes firing of EBNs and IBNs so that the agonist muscle stops contracting and the antagonist muscle contracts. At this point, omnipause neurons, which also receive inputs from FOR (not shown), may silence discharge of all EBNs and IBNs. C: As the second, leftward saccade starts, the left fastigial nucleus fires again to promote discharge of EBNs and IBNs. Note that early firing of the right fastigial nucleus may cause hypometria of the first saccade (Fig. 2B) or even prevent the first saccade from occurring (Fig. 3C).
BNs in the reticular formation of the medulla, pons and midbrain monosynaptically project the premotor signal for saccades to ocular motoneurons in cranial nerve nuclei III, IV and VI (Sparks, 2002;Horn, 2006). Both excitatory and inhibitory BNs exist and their projections ensure reciprocal innervation of agonist and antagonist extraocular muscles, in accordance with Sherrington’s law (Strassman et al., 1986a;Strassman et al., 1986b;Ramat et al., 2007). Initiation of saccades is strongly influenced by signals from the superior colliculus to BNs via intermediate long-lead BNs (Scudder et al., 1996) and to OPNs (Büttner-Ennever et al., 1999), which inhibit all BNs except during saccades. The cerebellum governs the activity of this brainstem network in order to make saccades accurate (Robinson & Fuchs, 2001). Thus, cerebellar disorders do not prevent saccades from being made but may cause saccades to become inaccurate and variable (Robinson et al., 1993;Leigh & Zee, 2006).
Studies in macaque monkey have identified two cerebellar areas that are important in the programming of saccades. The first is the dorsal vermis (lobules VI and VII); microstimulation here causes saccades with an ipsilateral component (Noda & Fujikado, 1987). Pharmacological inactivation or ablation of the dorsal vermis causes predominant ipsilateral hypometria and mild contralateral hypermetria of saccades (Sato & Noda, 1992;Takagi et al., 1998). The dorsal vermis projects to the fastigial nucleus (Yamada & Noda, 1987), and electrophysiological studies indicate that populations of Purkinje cells contributing to this projection encode the time when a saccade must stop to land on target (Thier et al., 2000). The caudal part of the fastigial nucleus – the fastigial ocular motor region (FOR) – receives input from the frontal eye fields and superior colliculus, via nucleus reticularis tegmenti pontis (NRTP), in addition to its projection from the dorsal vermis (Noda et al., 1990). The FOR influences saccades through its projections to inhibitory and excitatory BNs, and the OPNs (Noda et al., 1990;Robinson & Fuchs, 2001). FOR neurons discharge about 8 ms prior to onset of saccades with contralateral components, but towards the end of saccades with ipsilateral components (Fuchs et al., 1993). Unilateral pharmacological inactivation of the FOR causes hypermetria of ipsilateral saccades and hypometria of contralateral saccades (Robinson et al., 1993). Taken together, this evidence suggests that early activity in one FOR could be important for accelerating the eye at the beginning of a saccade, and the later activity in the other FOR could be critical for stopping the eye on target (Quaia et al., 1999;Robinson & Fuchs, 2001;Gad & Anastasio, 2010). For vertical saccades, a different circuit involving the posterior interpositus nucleus could provide a similar role to that which the FOR plays for horizontal saccades (Robinson, 2000).
What role could such a cerebellar network contribute to saccades made to double-step target jumps? In our experiments, the stimuli often required the horizontal component of the first and second saccade to reverse direction. We postulate that the FOR operates as a serial gate, accelerating the eye at the beginning of each saccade and stopping it at the movement’s end (Figure 8). For example, an initial rightward saccade would be promoted by early firing of the left FOR (Fig. 8A) and later firing of the right FOR (Figure 8B); for a subsequent, oppositely directed saccade, the order of FOR firing would reverse (Figure 8C). In the case of two saccadic commands reaching the FOR in close succession, there might be virtually no intersaccadic interval (OPNs remained silent), as one saccade ended and the next immediately started, corresponding to rapid reversal of the side of FOR firing. In normal subjects, this rapid reversal of the side of FOR firing may result in saccades directed to the first target of a double-step to become hypometric compared with responses to single target jumps (Fig. 3). We further postulate that, in the case of at least some of our cerebellar patients (especially CP1-3), their marked dysmetria (e.g., Figure 2A) could be due to abnormal timing of contralateral and ipsilateral FOR firing; this proposal is supported by the results of FOR inactivation summarized above (Robinson et al., 1993). Impaired FOR discharge might be responsible for the variability (no saccade, hypometria or hypermetria) found in cerebellar patients’ responses to DS1 (Figure 2B). A formal test of this hypothesis would call for presenting double-step stimuli with a range of durations between the first and second target jumps (interstimulus interval). Thus, a second target stimulus, opposite in direction to the first, might truncate the saccade made to the first target by disrupting the timing of the stop signal sent to the FOR.
Efference Copy and the Double-Step Task
Recent studies have supported the view that the ability to make accurate saccades to remembered target locations during the double-step task is dependent on a corollary discharge or efference copy of the motor command (i.e., an extraretinal signal) reaching the cortical eye fields (Wurtz & Sommer, 2004) from sources that include the cerebellum, via the ventrolateral nucleus of the thalamus (Noda et al., 1990;Bellebaum et al., 2005). Thus, three patients with cerebellar lesions were reported to show impaired accuracy of memory-guided saccades, which was ascribed to disruption of efference copy (Gaymard et al., 1994); however, this result was disputed (Kanayama et al., 1994;Bronstein et al., 1995). We found that, like control subjects, our 10 cerebellar patients made corrective saccades in darkness towards the remembered location of the target (Fig. 5), supporting the notion that an efference copy of eye movements, or a motor plan generated by the cerebral hemispheres (Goldberg & Bruce, 1990;Wurtz & Sommer, 2004), was still available, at least in the 10 patients we studied.
Conclusions and Clinical Implications
Taken together, the results of the present study indicate the important role of the cerebellum in governing sequences of saccades. Prior studies have applied the double-step stimulus paradigm to study the role of parietal cortex, frontal eye fields, and thalamic nuclei in saccade generation. However, any ability of the cerebral cortex to initiate or program a series of saccades is necessarily dependent on the brainstem machinery that generates these movements and the cerebellum, which governs their accuracy. Our findings indicate that the cerebellum might constitute a functional bottleneck in the ability to respond to a sequence of target jumps, especially if the cerebellum can only process one saccade at a time (at the level of the fastigial nuclei, Figure 8). More experiments are required to test this possibility.
To clinicians, inaccurate saccades, especially overshoots, are a hallmark of midline cerebellar disease affecting the dorsal vermis and deep nuclei. In this study, we identified another substantial deficit: cerebellar patients had difficulty in making a sequence of saccades between two rapidly presented visual targets. This apparent inability to carry out “parallel processing” of saccades has implications beyond eye movements and might well apply to limb movements (Wilmut et al., 2006) and gait. Thus, during locomotion in busy surroundings, the ability to adjust direction and balance in response to sudden environmental change can tax the ability of normal subjects. In cerebellar patients, who already have gait instability and abnormal vestibular responses, the sudden need to move in first one and then another direction could well lead to a fall. Such behavior might be analogous to the impaired ability to generate a sequence of saccades in responses to double-step target jumps. Our study indicates that cerebellar patients’ problems parallel processing further puts them at risk for injury when responding to rapid visual changes. Such behaviors are complex, depending on the integrity and integration of component motor systems. Studying saccades, for which the kinematic behavior is readily quantified and the neural substrate is defined, provides a powerful tool to investigate the nature of the cerebellar governance of complex movements.
Acknowledgements
Supported by Department of Veterans Affairs, National Institutes of Health grant R01-EY06717 and the Armington Fund (to Dr. Leigh). We are grateful to Dr. W. Michael King for his critical comments and to Drs. David Preston, Gregory Kosmorsky, and Bernd Remler for referring patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alahyane N, Fonteille V, Urquizar C, Salemme R, Nighoghossian N, Pelisson D, Tilikete C. Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum. 2008;7:595–601. doi: 10.1007/s12311-008-0065-5. [DOI] [PubMed] [Google Scholar]
- Becker W, Jurgens R. An analysis of the saccadic system by means of double step stimuli. Vision Res. 1979;19:967–983. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain. 2005;128:1139–1154. doi: 10.1093/brain/awh474. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Shallo-Hoffmann J, Kanayama R, Rudge P. Corrective saccades in cerebellar dysmetria. Ann Neurol. 1995;37:413–414. doi: 10.1002/ana.410370323. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AK, Henn V, Cohen B. Projections from the superior colliculus motor map to omnipause neurons in monkey. J Comp Neurol. 1999;413:55–67. doi: 10.1002/(sici)1096-9861(19991011)413:1<55::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Camalier CR, Gotler A, Murthy A, Thompson KG, Logan GD, Palmeri TJ, Schall JD. Dynamics of saccade target selection: race model analysis of double step and search step saccade production in human and macaque. Vision Res. 2007;47:2187–2211. doi: 10.1016/j.visres.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel JR, Goldberg ME, Fitzgibbon EJ, Sirigu A, Grafman J. Saccadic dysmetria in a patient with a right frontoparietal lesion. The importance of corollary discharge for accurate spatial behaviour. Brain. 1992;115(Pt 5):1387–1402. doi: 10.1093/brain/115.5.1387. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Bhutani N, Kulashekar S, Behari M, Goel V, Murthy A. Impaired conflict monitoring in Parkinson’s disease patients during an oculomotor redirect task. Exp Brain Res. 2010;208:1–10. doi: 10.1007/s00221-010-2432-y. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation: I. Neuronal discharge patterns. J Neurophysiol. 1993;70:1723–1740. doi: 10.1152/jn.1993.70.5.1723. [DOI] [PubMed] [Google Scholar]
- Gad YP, Anastasio TJ. Simulating the shaping of the fastigial deep nuclear saccade command by cerebellar Purkinje cells. Neural Netw. 2010;23:789–804. doi: 10.1016/j.neunet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Amarenco P, Pierrot-Deseilligny C. Influence of visual information on cerebellar saccadic dysmetria. Ann Neurol. 1994;35:108–112. doi: 10.1002/ana.410350117. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Primate frontal eye fields. III. Maintenance of a spatially accurate saccade signal. J Neurophysiol. 1990;64:489–508. doi: 10.1152/jn.1990.64.2.489. [DOI] [PubMed] [Google Scholar]
- Goossens HH, Van Opstal AJ. Local feedback signals are not distorted by prior eye movements: evidence from visually evoked double saccades. J Neurophysiol. 1997;78:533–538. doi: 10.1152/jn.1997.78.1.533. [DOI] [PubMed] [Google Scholar]
- Heide W, Blankenburg M, Zimmermann E, Kompf D. Cortical control of double-step saccades: implications for spatial orientation. Ann Neurol. 1995;38:739–748. doi: 10.1002/ana.410380508. [DOI] [PubMed] [Google Scholar]
- Horn AK. The reticular formation. In: Büttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. 2006. pp. 33–79. [PubMed] [Google Scholar]
- Iwamoto Y, Kaku Y. Saccade adaptation as a model of learning in voluntary movements. Exp Brain Res. 2010;204:145–162. doi: 10.1007/s00221-010-2314-3. [DOI] [PubMed] [Google Scholar]
- Joti P, Kulashekar S, Behari M, Murthy A. Impaired inhibitory oculomotor control in patients with Parkinson’s disease. Exp Brain Res. 2007;177:447–457. doi: 10.1007/s00221-006-0687-0. [DOI] [PubMed] [Google Scholar]
- Kanayama R, Bronstein AM, Shallo-Hoffmann J, Rudge P, Husain M. Visually and memory guided saccades in a case of cerebellar saccadic dysmetria. J Neurol Neurosurg Psychiatry. 1994;57:1081–1084. doi: 10.1136/jnnp.57.9.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SA, Chen AL, Joshi AC, Naassan G, Leigh RJ. Changing your mind: Cerebellar disorders and the double-step task. Soc Neurosci. 2010 Abstr 486.7. [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30:3715–3727. doi: 10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kori AA, Das VE, Zivotofsky AZ, Leigh RJ. Memory-guided saccadic eye movements: effects of cerebellar disease. Vision Res. 1998;38:3181–3192. doi: 10.1016/s0042-6989(98)00026-1. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements (Book/DVD) 4 ed. Oxford University Press; New York: 2006. Fourth Edition. [Google Scholar]
- MacAskill MR, Anderson TJ, Jones RD. Suppression of displacement in severely slowed saccades. Vision Res. 2000;40:3405–3413. doi: 10.1016/s0042-6989(00)00194-2. [DOI] [PubMed] [Google Scholar]
- Manto MU. Cerebellar Disorders. Cambridge University Press; New York: 2010. [Google Scholar]
- McLaughlin S. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362. [Google Scholar]
- McPeek RM, Skavenski AA, Nakayama K. Concurrent processing of saccades in visual search. Vision Res. 2000;40:2499–2516. doi: 10.1016/s0042-6989(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Noda H, Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol. 1987;58:359–378. doi: 10.1152/jn.1987.58.2.359. [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- Quaia C, Lefevre P, Optican LM. Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol. 1999;82:999–1018. doi: 10.1152/jn.1999.82.2.999. [DOI] [PubMed] [Google Scholar]
- Ramat S, Leigh RJ, Zee DS, Optican LM. What clinical disorders tell us about the neural control of saccadic eye movements. Brain. 2007;130:10–35. doi: 10.1093/brain/awl309. [DOI] [PubMed] [Google Scholar]
- Ramat S, Somers JT, Das VE, Leigh RJ. Conjugate ocular oscillations during shifts of the direction and depth of visual fixation. Invest Ophthalmol Vis Sci. 1999;40:1681–1686. [PubMed] [Google Scholar]
- Ray S, Schall JD, Murthy A. Programming of double-step saccade sequences: modulation by cognitive control. Vision Res. 2004;44:2707–2718. doi: 10.1016/j.visres.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson FR. Role of the cerebellar posterior interpositus nucleus in saccades I. Effect of temporary lesions. J Neurophysiol. 2000;84:1289–1302. doi: 10.1152/jn.2000.84.3.1289. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci. 2001;24:981–1004. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. Journal of Neurophysiology. 1993;70:1741–1758. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- Sato H, Noda H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis. Exp Brain Res. 1992;88:455–458. doi: 10.1007/BF02259122. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Moschovakis AK, Karabelas AB, Highstein SM. Anatomy and physiology of saccadic long-lead burst neurons recorded in the alert squirrel monkey .I. Descending projections from the mesencephalon. J Neurophysiol. 1996;76:332–352. doi: 10.1152/jn.1996.76.1.332. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3:952–964. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol. 1986a;249:337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol. 1986b;249:358–380. doi: 10.1002/cne.902490304. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- Thier P, Dicke PW, Haas R, Barash S. Encoding of movement time by populations of cerebellar Purkinje cells. Nature. 2000;405:72–76. doi: 10.1038/35011062. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Eye movement responses to a horizontally moving visual stimulus. Arch Ophthalmol. 1954;52:932–941. doi: 10.1001/archopht.1954.00920050938013. [DOI] [PubMed] [Google Scholar]
- Wilmut K, Wann JP, Brown JH. How active gaze informs the hand in sequential pointing movements. Exp Brain Res. 2006;175:654–666. doi: 10.1007/s00221-006-0580-x. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA. Identifying corollary discharges for movement in the primate brain. Prog Brain Res. 2004;144:47–60. doi: 10.1016/S0079-6123(03)14403-2. [DOI] [PubMed] [Google Scholar]
- Yamada J, Noda H. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol. 1987;265:224–241. doi: 10.1002/cne.902650207. [DOI] [PubMed] [Google Scholar]
- Young LR, Stark L. Variable feedback experiments testing a sampled data model for eye tracking movements. IEEE Trans Hum Factors Electron. 1963;HFE-4:38–51. [Google Scholar]
- Zee DS, Optican LM, Cook JD, ROBINSON DA, Engel WK. Slow saccades in spinocerebellar degeneration. Arch Neurol. 1976;33:243–251. doi: 10.1001/archneur.1976.00500040027004. [DOI] [PubMed] [Google Scholar]