Abstract
To study the immune response to prostate cancer, we developed an autochthonous animal model based on the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse in which spontaneously developing tumors express influenza hemagglutinin as a unique, tumor-associated antigen. Our prior studies in these animals showed immunologic tolerance to hemagglutinin, mirroring the clinical situation in patients with cancer who are generally nonresponsive to their disease. We used this physiologically relevant animal model to assess the immunomodulatory effects of cyclophosphamide when administered in combination with an allogeneic, cell-based granulocyte-macrophage colony-stimulating factor–secreting cancer immunotherapy. Through adoptive transfer of prostate/prostate cancer–specific CD8 T cells as well as through studies of the endogenous T-cell repertoire, we found that cyclophosphamide induced a marked augmentation of the antitumor immune response. This effect was strongly dependent on both the dose and the timing of cyclophosphamide administration. Mechanistic studies showed that immune augmentation by cyclophosphamide was associated with a transient depletion of regulatory T cells in the tumor draining lymph nodes but not in the peripheral circulation. Interestingly, we also noted effects on dendritic cell phenotype; low-dose cyclophosphamide was associated with increased expression of dendritic cell maturation markers. Taken together, these data clarify the dose, timing, and mechanism of action by which immunomodulatory cyclophosphamide can be translated to a clinical setting in a combinatorial cancer treatment strategy.
Introduction
Multiple immunotherapy strategies for prostate cancer are currently under preclinical and clinical evaluation, and several of these have advanced to the point of phase II or III trials (1–6). One of these strategies involves administration of irradiated, allogeneic tumor cells genetically modified to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF; ref. 7). This platform, known as GVAX (GM-CSF immunotherapy for cancer), appears to function via the secretion of GM-CSF by the modified tumor cells, which serves to recruit dendritic cells (DC) to the immunization site where they take up and process tumor antigens. Through the process known as cross-presentation, tumor antigens are then processed and presented to T cells by host antigen-presenting cells (8), potentially initiating an antitumor immune response.
Counteracting these immunotherapy strategies are a host of mechanisms by which evolving tumors escape immune recognition. Although considerable progress has been made toward understanding the mechanisms of immune evasion, T-cell tolerance to tumor-associated antigens appears to play a considerable role (8–11). To maximize the potential of cancer immunotherapy, it has been postulated that immune activation (“immunization”) will need to be combined with interventions designed to subvert the tolerogenic processes in tumor-bearing hosts (12). One relatively simple intervention by which the immune response to cancer immunotherapy may be augmented is through administration of low doses of the alkylating agent cyclophosphamide (13). This combination therapy has a long translational history, with initial studies done in the 1970s (14), and translation to the clinical setting by several groups (15, 16). Cyclophosphamide has been postulated to function in this setting through the depletion of Treg (13), a population of CD4+ T cells that serves to down-modulate an antitumor immune response in vivo (17–19).
The doses of cyclophosphamide used to augment an antitumor immune response are generally substantially lower than those used in standard clinical regimens, which are immunosuppressive by virtue of lymphodepletion. In addition, the relative timing of cyclophosphamide and the tumor immunotherapy under study is critical, as administration of the drug after immunization appears to dramatically impair immunotherapy response (13). However, the majority of these preclinical studies were done using models involving implanted tumor cells, a system that may or may not mimic the immune response to tumors in humans, as clinically relevant disease in patients may have evolved slowly over years. To more closely model the interaction between cell-based antitumor immunotherapy and administration of immunomodulatory cyclophosphamide, we used a murine system based on the transgenic adenocarcinoma of the mouse prostate (TRAMP) model, in which metastatic tumors arise progressively with age (20). By mating TRAMP mice with analogous transgenic mice that expresses the model antigen hemagglutinin (HA) in a prostate-restricted manner (ProHA; ref. 21), we created a model to study the immune response to tissue-tumor restricted HA as a function of immunotherapy and/or cyclophosphamide administration. As described herein, our results are broadly consistent with previous but suggest that a dose of cyclophosphamide lower than previously reported may be sufficient to augment antitumor responses. We also report novel data supporting a role for cyclophosphamide in multiple dosing regimens, showing that low-dose cyclophosphamide can be given before each immunization. In addition to a treatment benefit from the combined regimen, we also investigate the mechanism of action, showing that cyclophosphamide alters the cell surface phenotype of antigen-presenting cells at the immunization site in addition to depleting Treg at the tumor site.
Materials and Methods
Mice
Clone 4 is a CD8 TCR transgenic mouse recognizing (542IYST-VASSL550) in a Kd-restricted manner (22). Clone 4 mice were backcrossed over 12 generations onto a Thy1.1 congenic B10.D2 background. ProHA transgenic mice express secreted HA under control of the prostate epithelial-specific Probasin promoter (21) and are on a B10.D2 background. Double transgenic (ProHA × TRAMP) mice were generated by backcrossing TRAMP animals onto the ProHA background >12 generations. Double transgenic animals develop autochthonous prostate tumors expressing HA as a tissue/tumor-restricted antigen. Nontransgenic B10.D2 (H-2d) mice were purchased from The Jackson Laboratory. Mouse care and experimental procedures were carried out in accordance with the Institutional Animal Care and Use Committee of Johns Hopkins University under an approved protocol.
Cell lines
TRAMP-C2 was purchased from the American Type Culture Collection and maintained in DMEM with 5% fetal bovine serum, 5% NuSerum (Becton Dickinson), 10 nmol/L dihydrotestosterone (Sigma), and 5 μg/mL insulin (Sigma). B78H1-GM is a GM-CSF-secreting line (23), which has been described previously (24). These cells secrete ~2,500 ng GM-CSF/106 cells/24 h as determined by ELISA. To model HA-targeted immunotherapy, TRAMP-C2 cells were transfected with full-length HA as described previously (25), using Lipofectamine, according to the manufacturer’s instructions (Invitrogen). Transfectants were cloned by limiting dilution and HA expression was confirmed by staining with the HA-specific monoclonal antibody H18L10-5R1 (26), a gift of Dr. J. Yewdell. A single clone expressing high levels of HA was selected, expanded, and used in further studies.
Chemotherapy
Cyclophosphamide was purchased from Bristol-Myers Squibb and diluted in PBS for intraperitoneal injection.
Adoptive T-cell transfer
Adoptive T-cell transfer was done as described previously (27). Donor TCR transgenic mice were euthanized via CO2 asphyxiation. Spleens and lymph nodes were collected and homogenized, and RBC were lysed. CD8 T cells were purified using Miltenyi beads according to the manufacturer’s protocol. For some experiments, purified cells were labeled for 8 min with CFSE (Invitrogen) by adding 0.5 μL of 5 mmol/L stock per 1 mL cells. After labeling, cells were washed twice and resuspended in HBSS for intravenous injections. Cells (2.5 × 106) were injected per mouse in 0.2 mL total volume by tail vein injection.
Bystander immunotherapy (T-GVAX)
To model GVAX immunotherapy using bystander cells, 1 × 106 TRAMP-C2HA cells were admixed with 5 × 104 B78H1-GM cells and irradiated (5,000 rad). After washing thrice in HBSS, cells were resuspended in a total of 200 μL HBSS and administered by subcutaneous injection of 100 μL into each hind limb.
Flow cytometry
Prostate glands, prostate draining lymph nodes (DLN), and spleens were harvested on indicated days and single-cell suspensions were prepared. All staining reagents were from BD Pharmingen, with the exception of FoxP3 (eBioscience). After 10 min incubation, samples were washed once in PBS + 1% fetal bovine serum solution and analyzed using a FACSCalibur instrument (BD). Intracellular cytokine analysis was done as described previously (21, 27). Data were analyzed using the FlowJo software package (Treestar).
In vivo CTL assay
This assay was done as described previously (28). Splenocytes from naive B10.D2 mice were labeled with 2.5 or 0.25 μmol/L CFSE (Molecular Probes). CFSE-labeled cells (2.5μmol/L) were loaded with HA class I peptide (2μmol/L), whereas CFSE-labeled cells (0.25μmol/L) were used as a negative control. Target cells were transferred intravenously (7.5 × 106 cells of each population) into indicated groups of mice. Eighteen hours later, lymphocytes were isolated from spleen and fluorescence-activated cell sorting analysis was done. Histogram plots were used to determine the percentage of each target population based on intensity of CFSE staining. Percentage specific killing was calculated as described previously (29).
Treatment studies
Treatment was initiated at ages 7 to 9 weeks corresponding to an early intervention protocol (30). To model multiple treatments, immunization was repeated three additional times, 1 week apart. Mice were euthanized at ages 17 to 19 weeks. The urogenital organs were microdissected under a stereomicroscope and weighed. Ventral prostate lobes were removed and fixed in 10% neutral buffered formalin followed by 70% ethanol before paraffin embedding. Four micron sections were cut using a cryostat and placed onto poly-lysine-coated slides. Tissues were processed and stained with H&E, and tumor tissues were graded in a blinded manner by two individual pathologists as described previously (see Supplementary Fig. S1; ref. 27). Tumor tissues were also staged according to the extent of involvement: 1, focal; 2, multifocal; and 3, diffuse. Tumor score was calculated by tumor grade × tumor extent.
Lymphocyte counts
The TruCount system was used to quantify leukocytes according to the manufacturer’s instructions (BD). Directly conjugated monoclonal antibodies were added to TruCount tubes as follows: Lin cocktail, CD8-FITC, CD19-PE, CD4-APC, and CD45-PerCP. Anticoagulated whole peripheral blood (50μL) was added to 450μL FACS Lysing Solution, admixed, and added to tubes. Flow cytometry was done using a FACSCalibur instrument; absolute leukocyte count was calculated as (number of events in cell count region × number of beads per test)/(number of events in absolute bead count region × test volume).
In vitro suppression assay
These assays were done as described previously (31), and 3.5 × 104 purified T cells (responders) were admixed with increasing numbers of CD4+CD25+ T cells (suppressors) isolated from treated mice. Cells were incubated in round-bottomed 96-well tissue culture plates in 200μL CTL medium. Forty-eight to 72 hours later, cultures were pulsed with 1μCi [3H]thymidine and incubated an additional 16 h before harvest with a Packard Micromate cell harvester followed by counting with a Packard Matrix 96 direct β counter (Packard Biosciences).
Statistical analyses
Unless otherwise indicated, each experiment was done in triplicate using a minimum of five animals per group; representative results are shown. Mean ± SE is shown. For comparisons between groups, the one-way ANOVA with post-test comparison was done. Differences were considered statistically significant when the P < 0.05. Calculations were done using the GraphPad PRISM package (GraphPad).
Results
Tolerance to GVAX in tumor-bearing ProHA × TRAMP mice
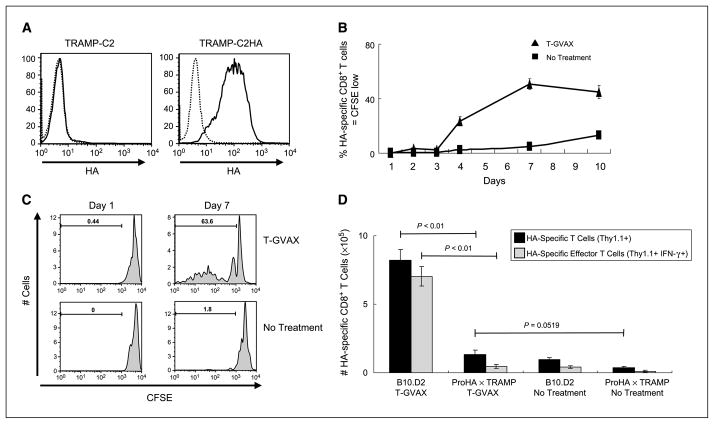
To model allogeneic prostate GVAX immunotherapy (7), we used the TRAMP-C2 prostate cancer cell line established from TRAMP mice (32), which is allogeneic with respect to the treated animals. TRAMP-C2 cells were transfected with full-length HA and HA expression was confirmed by fluorescence-activated cell sorting (Fig. 1A). For immunotherapy studies, this cell line (TRAMP-C2HA) was used in combination with a GM-CSF–secreting bystander cell line (B78H1-GM) as described previously (24). In vivo efficacy of this immunotherapy was confirmed in nontransgenic mice by adoptive transfer of CFSE-labeled HA-specific CD8 T cells followed by immunization. As shown in Fig. 1B and C, T-GVAX immunotherapy resulted in robust CD8 T-cell division, with a peak in division ~7 days postadministration. Intracellular staining confirmed that adoptively transferred HA-specific CD8 T cells responding to T-GVAX developed an effector phenotype; nearly all of the HA-specific divided cells in nontransgenic B10.D2 mice produced IFN-γ as assayed by intracellular staining (Fig. 1D). However, when identical cells were adoptively transferred to tumor-bearing ProHA × TRAMP mice, neither expansion nor cytokine production were detected following T-GVAX administration, consistent with our previous data showing tolerance to HA in these animals (21) as well as with data from other groups showing T-cell tolerance in mice with autochthonous prostate tumors (33–36).
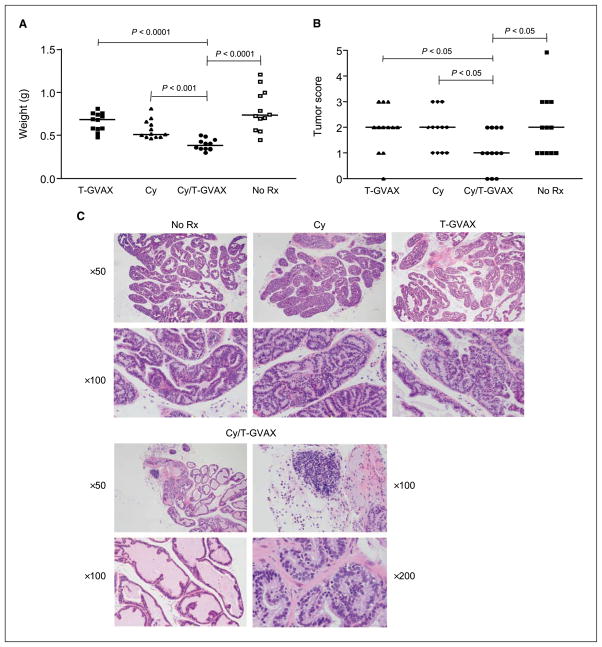
Figure 1.
Tolerance to GVAX in tumor-bearing ProHA × TRAMP mice. A, expression of HA by transfected TRAMP-C2 cells. Dotted line, isotype control. B, immunogenicity of T-GVAX. TRAMP-C2-HA cells were admixed with GM-CSF–secreting bystanders (T-GVAX) and administered intradermally 2 d after adoptive transfer of CFSE-labeled, HA-specific CD8+ T Cells. Peripheral blood cells from tail vein were harvested on indicated days postimmunization. Data are gated on HA-specific CD8+ Thy1.1+ lymphocytes that divided at least once. Mean ± SE. Three animals per group, representative of two experiments. C, T-GVAX-mediated proliferation. As above, CFSE plots gated on clonotypic (CD8+ Thy1.1+) T cells. D, tolerance to T-GVAX in tumor-bearing mice. CFSE-labeled HA-specific CD8+ T cells were adoptively transferred to indicated mice, and animals were treated 2 d posttransfer with T-GVAX immunotherapy. Seven days posttreatment, splenocytes were harvested and counted, and IFN-γ was quantified by intracellular staining. Three animals per group, representative of two experiments.
Timing and dosage of cyclophosphamide to augment T-GVAX function
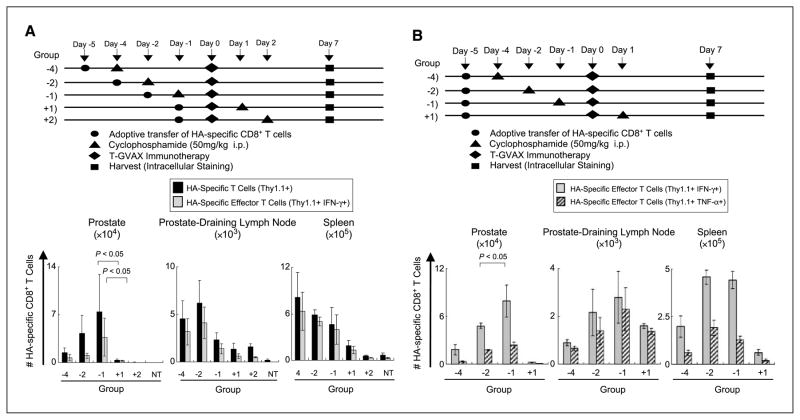
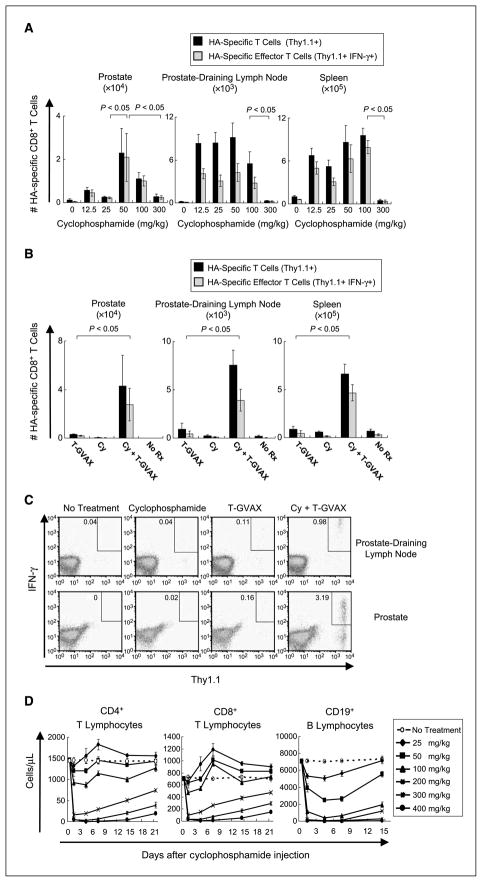
Because a single T-GVAX treatment was not sufficient to expand or induce an effector phenotype in prostate/prostate cancer–specific CD8 T cells transferred to tumor-bearing mice, we tested whether the addition of low-dose cyclophosphamide could affect T-cell tolerance in this model. Because previous studies showed that the timing of cyclophosphamide administration was critical (13), we varied the temporal relationship between cyclophosphamide and T-GVAX administration as shown in Fig. 2A and B. In initial studies, treatment was initiated after a 24 h in vivo rest, and function was assayed at a fixed (7 day) interval after T-GVAX immunotherapy. As shown in Fig. 2A, cyclophosphamide appeared to significantly augment T-GVAX–mediated CD8 T-cell expansion when given before immunotherapy administration. We further explored the relative timing of cyclophosphamide and T-GVAX using a second experimental design in which CD8 T cells remained in vivo for a fixed period in all experimental groups (Fig. 2B). This second set of studies confirmed the first, suggesting that a maximal organ-specific CD8 effector response occurred when cyclophosphamide is administered 1 day before T-GVAX. Given the presumably localized activity of effector CD8 T cells, we chose to administer cyclophosphamide at day −1 for further studies. We next explored the dose of cyclophosphamide used to augment CD8 T-cell number and function in tumor-bearing animals. As shown in Fig. 3A, an intraperitoneal dose of 50 mg/kg was optimal in this regard, with differences in specific T-cell expansion and effector phenotype most apparent in the prostate gland itself. Compared with either T-GVAX or cyclophosphamide alone, these results were significant, with nearly an order of magnitude increase in the number of HA-specific CD8 T cells in both the prostate and the prostate DLN (Fig. 3B and C). Similar increases in tumor necrosis factor-α, granzyme B, and perforin secretion were noted with the combined treatment regimen as well (Supplementary Fig. S3). Intravenous administration of cyclophosphamide was not effective in this regard (data not shown). As metabolism of pharmacologic agents can be different in the mouse compared with the human, we determined the biological effects of these low doses of cyclophosphamide in terms of lymphocyte counts using TruCount technology. As shown in Fig. 3D, the lower doses of cyclophosphamide that were effective in terms of increasing effector CD8 T-cell expansion did not result in significant depletion of either CD8 or CD4 T cells from the peripheral circulation. Interestingly, there was a fairly sharp demarcation in this regard, with doses >100 mg/kg showing relatively prolonged T-cell depletion in these mice. In addition, the optimal dose of cyclophosphamide in terms of T-cell expansion (50 mg/kg) resulted in a significant reduction in the number of circulating B cells, possibly consistent with prior studies suggesting a potential role for B cells in mediating tolerance in certain models (37).
Figure 2.
Timing of cyclophosphamide + T-GVAX. A, timing of immunomodulatory cyclophosphamide with fixed in vivo rest. Top, experimental design. Treatment initiated 24 h post–adoptive transfer in all groups. Bottom, tumor-bearing animals were sacrificed on day +7 postimmunotherapy, with indicated cyclophosphamide timing. HA-specific CD8+ T cells were quantified from indicated sites and evaluated for IFN-γ and tumor necrosis factor-α secretion by intracellular staining. Three animals per group, representative of two experiments. B, timing of cyclophosphamide with a fixed in vivo dwell time. Top, experimental design; bottom, HA-specific CD8+ T cells were quantified from indicated sites and evaluated for IFN-γ secretion by intracellular staining.
Figure 3.
Dosage of immunomodulatory cyclophosphamide. A, experiment performed as in Fig. 2A, with intraperitoneal cyclophosphamide dose varied as indicated. Three animals per group, representative of two experiments. B, immunologic effects of combined cyclophosphamide (Cy)/T-GVAX treatment. Eighteen- to 20-week-old (tumor-bearing) ProHA × TRAMP mice were treated day −1 with 50 mg/kg cyclophosphamide intraperitoneally followed by T-GVAX on day 0. Animals were harvested on day +7 and analyzed as above. Three animals per group, representative of two experiments. C, representative dot plots for panel B. D, effects of cyclophosphamide on circulating lymphocyte numbers. Animals were treated on day 0 with indicated cyclophosphamide dose and analyzed for indicated lymphocyte populations using TruCount technology. Three animals per group, representative of two experiments.
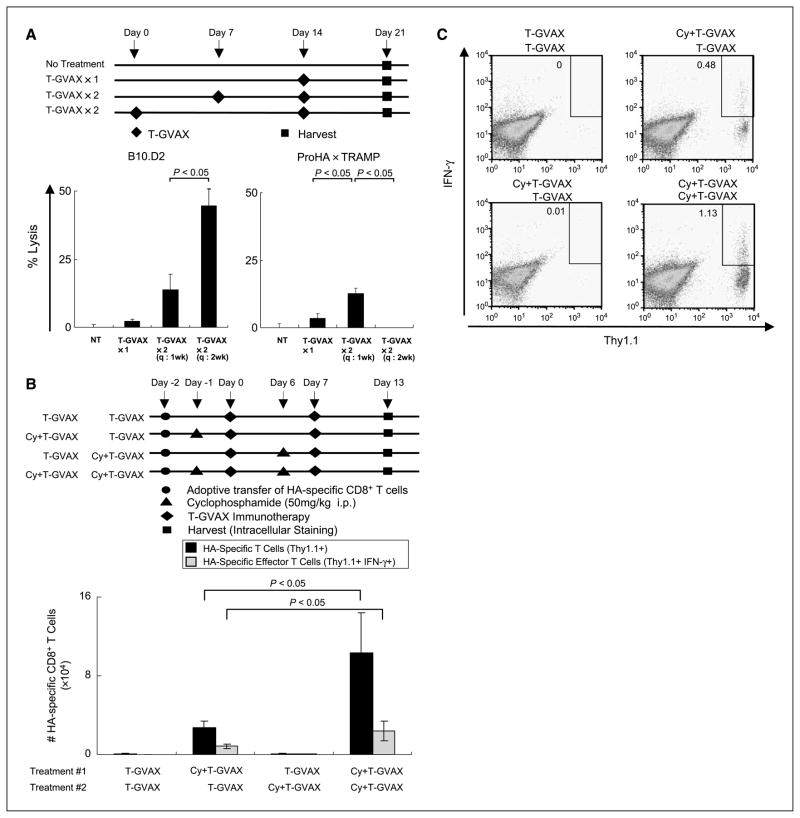
Cyclophosphamide in multidose treatment regimens
Because induction of an antitumor immune response typically requires multiple treatments (38), we examined the effects of combining cyclophosphamide with T-GVAX in multidose regimens. For these longer-term studies, the lifespan of adoptively transferred HA-specific CD8 T cells might prove limiting, as such cells are gradually eliminated in a tolerogenic environment (39). Thus, we chose initially to evaluate the function of the endogenous immune repertoire using an in vivo CTL as a readout (29). We first tested whether T-GVAX immunization could be boosted at a 1- or 2-week interval (Fig. 4A). These experiments were done in both non-transgenic (B10.D2) mice and 18- to 20-week-old tumor-bearing ProHA × TRAMP mice. In mice without autochthonous tumors, boosting at a 2-week interval proved optimal (Fig. 4A). However, this was not the case for tumor-bearing animals, where weekly immunotherapy resulted in a greater CTL response in vivo. Because this shorter interval was well within the expected persistence of adoptively transferred prostate/prostate-tumor specific CD8 T cells, we returned to the more sensitive adoptive transfer system to determine whether cyclophosphamide should be administered with each immunotherapy dose (Fig. 4B). To our surprise, the addition of low-dose (50 mg/kg) cyclophosphamide to both prime and boost immunotherapy doses resulted in maximal persistence of adoptively transferred CD8 T cells (Fig. 4B and C). The effects of ongoing tolerogenic processes were notable, as a lower percentage of adoptively transferred T cells secreted IFN-γ at this later time point. Despite this, the number of prostate/prostate cancer-specific effector cells was significantly increased when cyclophosphamide was administered before each T-GVAX administration.
Figure 4.
Multidose studies. A, endogenous CTL function. Top, experimental design. Bottom, CTL function in B10.D2 (left) and ProHA × TRAMP (right) animals. Three animals per group, representative of two experiments. B, cyclophosphamide + T-GVAX in multidose regimen. HA-specific CD8+ T cells were adoptively transferred on day −2 to ProHA × TRAMP mice. Prostate glands were harvested on day +13 after initial treatment and evaluated for IFN-γ secretion by intracellular staining. Three animals per group, representative of two experiments. C, representative dot plots for panel B.
Combining T-GVAX with low-dose cyclophosphamide: treatment effects
To test for an additive antitumor effect of the combined regimen, ProHA × TRAMP mice ages 7 to 9 weeks were treated a total of four times with immunotherapy: (T-GVAX) alone, cyclophosphamide alone, or the combination. Ten weeks after initial treatment, animals were sacrificed and tumor extent was evaluated as described previously (27). As shown in Fig. 5A and Supplementary Fig. S2, the wet weight of the urogenital tract, a gross surrogate for tumor burden (40), was significantly decreased in the combination treatment group compared with either single treatment alone. T-GVAX immunotherapy alone had no treatment effect in this autochthonous model, whereas cyclophosphamide alone showed a nonsignificant trend toward efficacy compared with untreated control animals. Blinded scoring of the micro-dissected ventral lobe of the prostate gland corroborated these data, again showing a significant decrease in tumor score in the combined treatment group compared with either single treatment alone (Fig. 5B). Interestingly, lymphocytic infiltration and apoptotic bodies were more frequently noted in the combined treatment group (Fig. 5C).
Figure 5.
Antitumor effect of combined regimen. A, wet weight of the urogenital tract. Twelve animals per group. B, tumor score of treated animals. Tumor score computed by multiplying pathologic score × extent (see Materials and Methods). Twelve animals per group. C, histologic evaluation of treated animals. Representative H&E-stained sections.
Mechanism of combined treatment effect
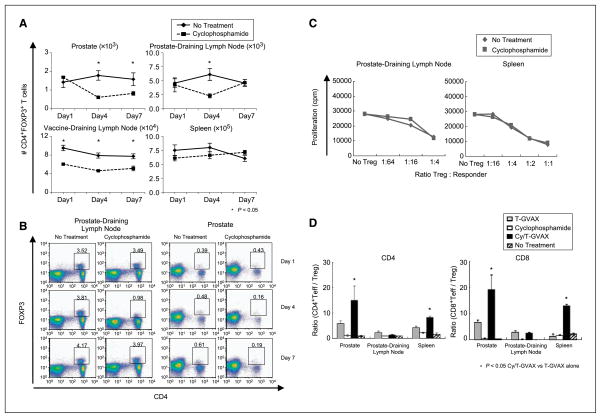
Early studies suggested that the immunomodulatory effects of low-dose cyclophosphamide were mediated through the depletion of regulatory T cells (Treg; ref. 41). We thus quantified Treg in the various sites in tumor-bearing ProHA × TRAMP mice ages 16 to 18 weeks following treatment with low-dose T-GVAX + cyclophosphamide. As shown in Fig. 6A and B, a relative Treg depletion was noted at the tumor site (prostate gland), but not in the spleen, which more closely approximates the peripheral circulation. The prostate DLN also showed a relative Treg depletion, but this effect was transient, with relative Treg infiltration returning to normal levels by day +7 posttreatment. Decreased absolute numbers of Treg were also noted in the vaccine DLN. Treg depletion in these studies did not appear to involve a selective induction of apoptosis in this population, as increased Annexin-5 staining in Treg was not noted after cyclophosphamide administration (Supplementary Fig. S4). In addition to these quantitative effects, it was possible that the treatment effect of cyclophosphamide could be mediated by a relative decrease in Treg function as well as number. Thus, we performed classic in vitro suppression assays using Treg sorted from the spleen or prostate DLN 4 days post–cyclophosphamide administration. As shown in Fig. 6C, Treg from both cyclophosphamide-treated and control animals mediated suppression equally well in these studies, suggesting that the immune effects of cyclophosphamide on Treg are mediated by decreases in Treg number rather than by alterations in Treg function. Recent studies suggested that treatment effects in mice treated with a combination of GVAX and anti-CTLA4 seemed to correlate with increases in the ratio of effector cells to Treg (42). Thus, we evaluated the relative ratio of CD4+ and CD8+ effectors to Treg under the various treatment conditions. As shown in Fig. 6D, the combined treatment resulted in an increase in the ratio of CD4+ or CD8+ effector T cells/Treg in the prostate gland but not in the prostate DLN. The combination of cyclophosphamide and T-GVAX also appeared to have a significant effect in the Teff/Treg ration in the spleen, suggesting a potential parameter for peripheral monitoring. In addition to effects on Treg, low-dose cyclophosphamide may affect either the number or the maturation state of DC, which serve as antigen-presenting cells. Therefore, we examined the number and cell surface phenotype of DC in various sites. We evaluated both CD8+CD11c+ DC, the primary population involved in cross-presentation (43), and the CD8− population, which may present antigen when activated (44). As shown in Supplementary Fig. S5, the combination of cyclophosphamide and T-GVAX appeared to increase the number of CD8− DC in the prostate DLN compared with T-GVAX alone, but the number of CD8− DC was decreased at the vaccine DLN. Absolute numbers of CD8+ (cross-presenting) DC were not increased with the combination treatment in any of the sites examined. These data suggest that the combinatorial effect of T-GVAX + low-dose cyclophosphamide are most likely not mediated by dramatic changes in DC number. However, the combination of cyclophosphamide and T-GVAX did appear to induce a more proinflammatory DC phenotype in both CD8− (Supplementary Fig. S6) and CD8+ (Supplementary Fig. S7) populations. Notably, a significant increase in the mean fluorescence intensity of class II MHC on CD8+ DC was noted in the prostate DLN with the combination treatment compared with T-GVAX alone (Supplementary Fig. S7). Taken together, these data support the notion that depletion of Treg may be a major mechanism of action of low-dose cyclophosphamide in this combined treatment regimen but that a more proinflammatory DC phenotype may contribute as well.
Figure 6.
Effects of low-dose cyclophosphamide + T-GVAX on Treg number and function. A, Treg depletion. Following low-dose cyclophosphamide administration, absolute number of CD4+ T cells = FoxP3+ quantified using intracellular staining on indicated days. n = 3 animals per group, repeated × 2. B, representative dot plots for panel A. C, Treg function. Treg (CD4+CD25+CD62Lhigh) isolated from DLN or spleens of indicated mice using magnetic bead sorting. In vitro suppression assay was done using 3.5 × 104 suppressors and 3.5 × 104 responders (ratio 1:1). Proliferation quantified by H3 incorporation. Three animals per group, representative of three experiments. D, ratio Teff/Treg. CD4+ and CD8+ effector T cells (IFN-γ–positive) were quantified on day +14 following administration of T-GVAX + cyclophosphamide, and compared with Treg, quantified using FoxP3+ intracellular staining as above. Three animals per group, representative of two experiments.
Discussion
In these studies, we evaluated the effects of adding low-dose cyclophosphamide to a cell-based immunotherapy in mice bearing endogenous prostate tumors. Although the immunomodulatory effects of cyclophosphamide have been previously evaluated (13), to our knowledge, these studies represent the first attempt to understand this interaction in an autochthonous tumor model. In terms of timing, our data provide strong confirmation of earlier work, suggesting that administration of cyclophosphamide 1 or 2 days before GVAX immunotherapy treatment provides an optimal effect. It should be noted that our studies were limited to a single tumor immunotherapy modality (GVAX) and that the timing of cyclophosphamide treatment relative to immunization may vary with the immunotherapy construct employed. We also carefully evaluated the relative dosage of cyclophosphamide required for a combined treatment effect; our data suggest that the optimum dose is slightly lower than previous data would indicate (13). However, the cyclophosphamide dosage examined here (50 mg/kg intraperitoneally) is within the range of earlier studies suggesting an optimal dose of 100 mg/kg and may reflect slight differences in immunotherapy potency in the models examined or potentially differences in the models themselves.
We also evaluated the effects of combined treatment in a multidose setting that more accurately resembles current clinical treatment paradigms. To our knowledge, these data are novel and suggest that low-dose cyclophosphamide treatment might prove optimal when administered before each immunotherapy dose. These data have important clinical implications and are consistent with recently published data showing a cumulative effect on Treg depletion over time in patients treated with oral cyclophosphamide (5). Interestingly, others have been able to detect decreases in Treg number in the periphery of treated patients, an effect that does not seem to be mirrored in our shorter-term animal studies, where we found that the effects of low-dose cyclophosphamide on Treg number were relatively confined to the tumor itself as well as to the tumor DLN. It should be further noted that the effects of cyclophosphamide in this model were achieved with intraperitoneal versus intravenous administration. The mechanism behind this differential effect is currently not known, but the efficacy of intraperitoneal administration (which involves multicompartment pharmacokinetics) might support the concept of oral administration of cyclophosphamide to cancer patients as has been suggested by a recent clinical trial (5).
We also evaluated the mechanism of action of low-dose cyclophosphamide in this setting. As above, our results suggest Treg depletion as a major mechanism. However, low-dose cyclophosphamide alone did show a trend toward a treatment effect in ProHA × TRAMP mice, suggesting either a direct anti-tumor effect or, perhaps more interestingly, an effect on Treg in the absence of specific immunization. It should be noted that separating a Treg-depleting effect from other possible mechanisms of action is difficult, because available alterative strategies are incapable of completely depleting Treg to provide an appropriate control (45). To this end, we also studied the effects of low-dose cyclophosphamide on DC number and function. Although the numbers of CD8+ or CD8− DC were generally not increased by combination treatment, both populations appeared to show an increased mean fluorescence intensity of class II MHC in the prostate DLN, consistent with a more activated DC phenotype. These findings are consistent with recent studies suggesting that Treg may function in vivo by altering DC phenotype (46).
In summary, these data provide strong experimental support for a strategy combining low-dose cyclophosphamide with allogeneic GVAX immunotherapy. This concept has a long history, with initial studies combining a melanoma vaccine with cyclophosphamide done in the 1980s (47). This combination has also been evaluated with other cancer vaccines (48), and a recent study using GVAX alone versus low-dose cyclophosphamide + GVAX in patients with pancreatic cancer supports the clinical utility of our results (49). Although the majority of such studies have used a fixed dose of cyclophosphamide, a recent trial in women with breast cancer is consistent with the data presented here; at increasing cyclophosphamide doses, combinatorial effects were lost (50). As such, our data showing that small increases in cyclophosphamide dose can result in a loss immunotherapy efficacy are consistent with the notion that clinical trials using this combined approach should consider a dose-titration phase. In addition, our multidose modeling suggested that cyclophosphamide should be administered before each immunotherapy cycle. Most critically, it should be noted that the treatment effects of the combined regimen are fairly striking, especially in light of the significant tumor-specific tolerance we have previously shown in this model.
Supplementary Material
Acknowledgments
Grant support: Cell Genesys sponsored research agreement, NIH grants R01 CA127153 and K08 CA096948 (C.G. Drake), and Patrick C. Walsh Fund.
Footnotes
C.G. Drake is a Damon Runyon Clinical Fellow.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
C.G. Drake: commercial research support, Cell Genesys, Inc. The other authors disclosed no potential conflicts of interest.
References
- 1.Drake CG. Immunotherapy for metastatic prostate cancer. Urol Oncol. 2008;26:438–44. doi: 10.1016/j.urolonc.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeel DG. Prostate cancer immunotherapy. Curr Opin Urol. 2007;17:175–81. doi: 10.1097/MOU.0b013e3280eb10eb. [DOI] [PubMed] [Google Scholar]
- 3.Fong L, Small EJ. Immunotherapy for prostate cancer. Curr Oncol Rep. 2007;9:226–33. doi: 10.1007/s11912-007-0026-z. [DOI] [PubMed] [Google Scholar]
- 4.Tarassoff CP, Arlen PM, Gulley JL. Therapeutic vaccines for prostate cancer. Oncologist. 2006;11:451–62. doi: 10.1634/theoncologist.11-5-451. [DOI] [PubMed] [Google Scholar]
- 5.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlen PM, Kaufman HL, DiPaola RS. Pox viral vaccine approaches. Semin Oncol. 2005;32:549–55. doi: 10.1053/j.seminoncol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24:419–24. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–68. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–61. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 11.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 12.Pardoll D, Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat Med. 2004;10:887–92. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- 13.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 14.Maguire HC, Jr, Ettore VL. Enhancement of dinitrochlorobenzene (DNCB) contact sensitization by cyclophosphamide in the guinea pig. J Invest Dermatol. 1967;48:39–43. doi: 10.1038/jid.1967.6. [DOI] [PubMed] [Google Scholar]
- 15.Emens LA, Armstrong D, Biedrzycki B, et al. A phase I vaccine safety and chemotherapy dose-finding trial of an allogeneic GM-CSF-secreting breast cancer vaccine given in a specifically timed sequence with immunomodulatory doses of cyclophosphamide and doxorubicin. Hum Gene Ther. 2004;15:313–37. doi: 10.1089/104303404322886165. [DOI] [PubMed] [Google Scholar]
- 16.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer—science driving clinical progress. Nat Rev Cancer. 2005;5:459–67. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 17.von BH. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 18.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan DJ, Liblau R, Scott B, et al. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–83. [PubMed] [Google Scholar]
- 23.Borrello I, Sotomayor EM, Cooke S, Levitsky HI. A universal granulocyte-macrophage colony-stimulating factor-producing bystander cell line for use in the formulation of autologous tumor cell-based vaccines. Hum Gene Ther. 1999;10:1983–91. doi: 10.1089/10430349950017347. [DOI] [PubMed] [Google Scholar]
- 24.Luznik L, Slansky JE, Jalla S, et al. Successful therapy of metastatic cancer using tumor vaccines in mixed allogeneic bone marrow chimeras. Blood. 2003;101:1645–52. doi: 10.1182/blood-2002-07-2233. [DOI] [PubMed] [Google Scholar]
- 25.Staveley-O’Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy:anearlyevent in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–83. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berglund P, Finzi D, Bennink JR, Yewdell JW. Viral alteration of cellular translational machinery increases defective ribosomal products. J Virol. 2007;81:7220–9. doi: 10.1128/JVI.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8 T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–92. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]
- 30.Huss WJ, Maddison LA, Greenberg NM. Autochthonous mouse models for prostate cancer: past, present and future. Semin Cancer Biol. 2001;11:245–60. doi: 10.1006/scbi.2001.0373. [DOI] [PubMed] [Google Scholar]
- 31.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30. [PubMed] [Google Scholar]
- 33.Degl’Innocenti E, Grioni M, Boni A, et al. Peripheral T cell tolerance occurs early during spontaneous prostate cancer development and can be rescued by dendritic cell immunization. Eur J Immunol. 2005;35:66–75. doi: 10.1002/eji.200425531. [DOI] [PubMed] [Google Scholar]
- 34.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–8. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178:1268–76. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 36.Lees JR, Charbonneau B, Hayball JD, et al. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate. 2006;66:578–90. doi: 10.1002/pros.20307. [DOI] [PubMed] [Google Scholar]
- 37.Jamin C, Morva A, Lemoine S, Daridon C, de Mendoza AR, Youinou P. Regulatory B lymphocytes in humans: a potential role in autoimmunity. Arthritis Rheum. 2008;58:1900–6. doi: 10.1002/art.23487. [DOI] [PubMed] [Google Scholar]
- 38.Su Z, Dannull J, Yang BK, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with meta-static prostate cancer. J Immunol. 2005;174:3798–807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 39.Redmond WL, Hernandez J, Sherman LA. Deletion of naive CD8 T cells requires persistent antigen and is not programmed by an initial signal from the tolerogenic APC. J Immunol. 2003;171:6349–54. doi: 10.4049/jimmunol.171.12.6349. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan-Lefko PJ, Chen TM, Ittmann MM, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 41.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J Exp Med. 2002;196:817–27. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008;333:167–79. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–8. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berd D, Maguire HC, Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–7. [PubMed] [Google Scholar]
- 48.Holmberg LA, Sandmaier BM. Theratope vaccine (STn-KLH) Expert Opin Biol Ther. 2001;1:881–91. doi: 10.1517/14712598.1.5.881. [DOI] [PubMed] [Google Scholar]
- 49.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emens LA, Asquith JM, Leatherman JM, et al. Increasing doses of cyclophosphamide suppress antigen-specific T helper-dependent immunity induced by a GM-CSF-secreting breast tumor vaccine. J Clin Oncol. 2008;26:3009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.