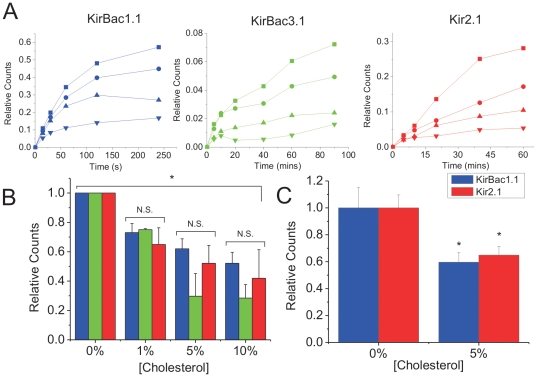
Figure 1. Cholesterol inhibits activity of reconstituted KirBac1.1, KirBac3.1 and human Kir2.1.
(A) Representative time course of 86Rb+ uptake into 9∶1 POPE∶POPG liposomes (+1% PI(4,5)P2 for Kir2.1) containing increasing amounts of cholesterol reconstituted (0% = ▪; 1% = •; 5% = ▴; 10% = ▾) with KirBac1.1 (left), KirBac3.1 (middle), or Kir2.1 (right) protein, incubated with 450 mM internal and 0 mM external [K+]. Uptake was normalized to valinomycin-induced uptake in the same liposomes. (B) Channel activity-cholesterol relationship obtained from 86Rb+ uptake at 240 s for KirBac1.1 (blue; n = 7±s.e.m), at 90 mins for KirBac3.1 (green; n = 6±s.e.m) and at 60 mins for human Kir2.1 (red; n = 6±s.e.m) for each cholesterol concentration. The data are renormalized to uptake in liposomes containing 0% cholesterol. (C) 86Rb+ uptake from KirBac1.1 (blue) and Kir2.1 (red) channels reconstituted into liposomes containing 25% POPG (+1% PI(4,5)P2 for Kir2.1) ±5% cholesterol on a POPE background (n = 8 for each). The data were normalized to valinomycin-induced uptake in the same liposomes and re-normalized to uptake in liposomes containing 0% cholesterol. (* P<0.05 assessed by ANOVA).