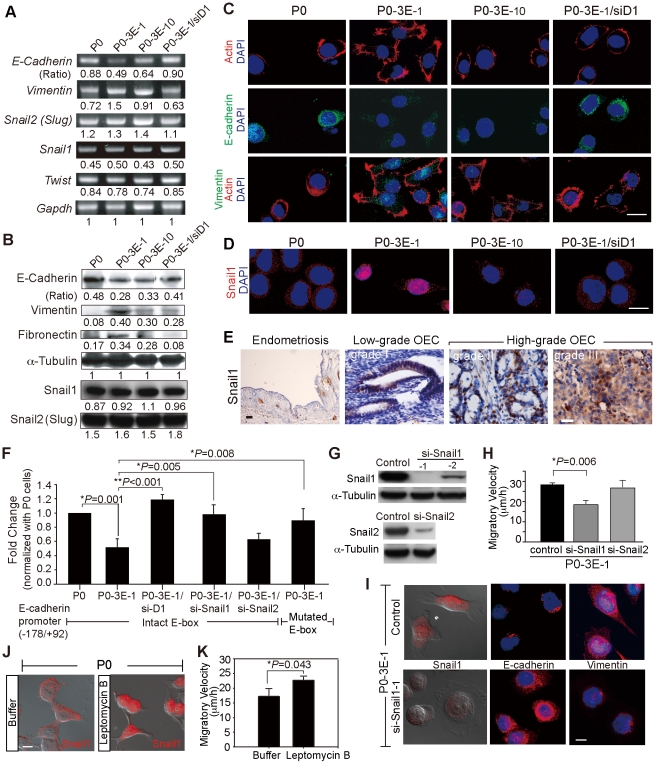
Figure 5. Sema3E/Plexin-D1 mediated epithelial-to-mesenchymal transition depends on nuclear translocation of Snail1.
A, B. Expression of cellular markers of EMT correlates with levels of Sema3E/Plexin-D1 activity in modified OEC cells as measured by semi-quantitative RT-PCR (A) and Western blotting (B). By contrast, members of the Snail family transcription factors that regulate developmental EMT are uniformly expressed in modified OEC cell lines. Signal intensities were normalized to Gapdh (A) or œ-Tubulin (B), respectively, and are indicated as ratios. C, D. Confocal microscopy of OEC cells shows a shift from epithelial (E-cadherin) to mesenchymal (Vimentin) marker expression and a distinct nuclear translocation of Snail1 in modified OEC cells exhibiting greater Sema3E/Plexin-D1 activity. Immunoreactivity for filamentous actin, and nuclear DAPI staining were used as general markers of cell morphology. Scale bar, 20 µm. E. Nuclear localization of Snail1 is associated with high grade of human OEC tissue, but not low-grade human OEC tissues. Snail1 was not detected in ovarian endometriosis. Scale bar, 10 µm. F. E-cadherin promoter activity, measured by normalized luciferase expression in modified OEC lines, is down-regulated in cells with high Sema3E/Plexin-D1 signaling. This is dependent on Snail1 translocation since knockdown of Snail1 or a mutated E-box prevents transcriptional repression. Error bars show s.e.m., n = 9. **, P<0.01, *, P<0.05, paired t-test. G. Western blots illustrate RNAi-mediated knockdown of Snail1 in Sema-3E expressing P0-3E-1 cells via lenti-viral infection. H. Knockdown of Snail1 expression in P0-3E-1 cells decreases migration in wound healing assay. I. Knockdown of Snail1 in P0-3E-1 cells induces a shift from spindle shaped, vimentin positive to a rounded, E-cadherin positive cellular morphology. Scale bar, 10 µm. J, K. Pharmacologically-induced retention of nuclear Snail1 by leptomycin B (20 ng/ml) increases migratory ability and induces a spindle shaped cellular morphology in P0 cells. Scale bar, 10 µm.