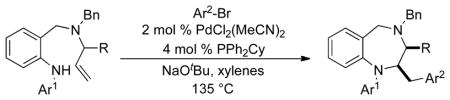
Table 2.
Synthesis of Saturated 1,4-Benzodiazepinesa
 | |||
|---|---|---|---|
| entry | substrate | product | yield (%)b |
| 1 |
 14b |
 17 |
78 |
| 2 | 14b |
 18 |
88 |
| 3 |
 14c |
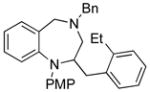 19 |
94 |
| 4 |
 14d |
 20 |
71 |
| 5 | 14d |
 21 |
74 |
| 6 |
 14e |
 22 |
82c |
| 7 |
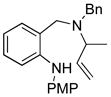 14a |
 15 |
65 |
| 8 | 14a |
 23 |
81c |
| 9 | 14a |
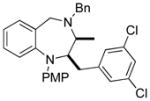 24 |
62 |
| 10 | 14a |
 25 |
84 |
| 11 | 14a |
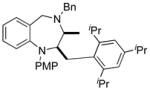 26 |
60 |
Conditions: Reactions were conducted on a 0.15 mmol scale using 1.0 equiv substrate, 2.0 equiv ArBr, 2.0 equiv NaOtBu, 2 mol % PdCl2(MeCN)2, 4 mol % PPh2Cy, xylenes (0.2 M), 135 °C, 18–24 h reaction time.
Isolated yield (average of two experiments). In all cases, 2,3-disubstituted products were obtained with >20:1 dr.
This product contained ca. 8% of ketone side product16.
