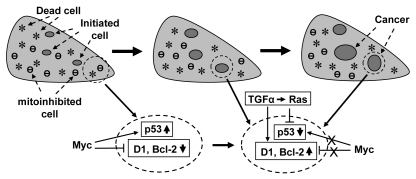
Figure 3.
Illustration of our hypothesis on how c-Myc-induced compensatory proliferation and mitoinhibition enhance carcinogenesis in transgenic animals: the c-myc transgene causes cell death (*) and probably also mitoinhibition (θ), which may be mechanistically related to its inhibition of cyclin D1 and other oncogenes (e.g., Bcl-2) as well as to its induction of p53 and other tumor suppressor genes, as shown in an enlarged area in the bottom part. The cell death triggers compensatory proliferation of the organ. Thus, cell proliferation is driven not only by the c-myc transgene per se but also by the cell death, but it still remains unknown which c-myc expressing cells, among many others, decide or are selected to die, to proliferate, or to be mitoinhibited. During the compensatory proliferation, some critical genetic changes occur sporadically in some cells, thus creating initiated cells that may be less mitoinhibitory and have a stronger survival potential, relative to the surrounding cells. The initiated cells proliferate continuously in a clonal expansion fashion and accumulate more and more genetic alternations, developing to preneoplastic lesions and, for some of them, malignant tumors eventually. During this process, some cells may develop mechanisms to escape from the control by c-Myc, resulting in p53 inactivation or D1 induction, as depicted in another enlarged area in the bottom part. At any time of the process, many growth stimuli such as TGFα EGF may collaborate, often via Ras, with c-Myc in the carcinogenesis in part by inducing D1 or inactivating the p53 pathway.