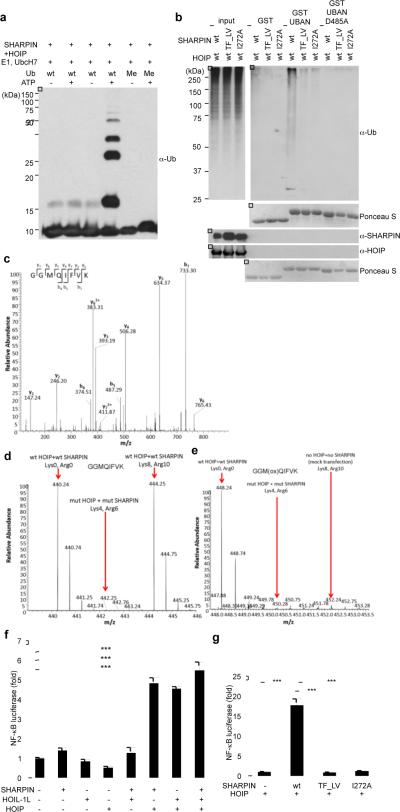
Figure 2. SHARPIN and HOIP form a novel LUBAC complex with the ability to induce linear ubiquitylation and NF-κB activation.
a, In vitro linear ubiquitin chain synthesis by purified SHARPIN and HOIP. b, Stimulation of linear ubiquitylation by SHARPIN and HOIP in vivo. Immobilised GST-ABIN-1-UBAN domain was used to detect linear ubiquitylation of proteins. c, MS/MS spectrum of the prototypic linear ubiquitin peptide present in vivo. d, e, SILAC experiments comparing relative levels of linear ubiquitin on immunoprecipitated NEMO induced by HOIP and SHARPIN (d) or HOIP-mut (RING domain)-SHARPIN-mut( TF_LV)(Lys8,Arg10) (e). f, g, Stimulation of NF-κB transcriptional activity by SHARPIN and HOIP in vivo. NF-κB-luciferase assays using the indicated combinations of SHARPIN, HOIL-1L, and HOIP were performed. Results are shown as means and s.e.m. (n=4). *P < 0.0001, determined by the Student's t-test