Table 1.
Activity of quinoline urea analogues against OX1R and OX2R.
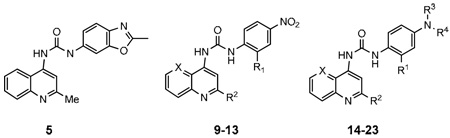 | |||||||
|---|---|---|---|---|---|---|---|
| No. | R1 | R2 | R3 | R4 | X | Ke (nM) | |
| OX1a | OX2b | ||||||
| 1 | 45±12 | 1194 | |||||
| 5 | 682.6±231 | >10,000 | |||||
| 9 | H | H | NO2 | N | >10,000 | nt | |
| 10 | H | Me | NO2 | CH | >10,000 | nt | |
| 11 | Cl | Me | NO2 | CH | >10,000 | nt | |
| 12 | Me | Me | NO2 | CH | >10,000 | nt | |
| 13 | OMe | Me | NO2 | CH | >10,000 | nt | |
| 14 | H | Me | H | H | CH | >10,000 | >10,000 |
| 15 | H | Me | Me | Me | CH | 356.7±89 | >10,000 |
| 16 | Me | Me | Me | Me | CH | 2561±266 | >10,000 |
| 17 | OMe | Me | Me | Me | CH | 3945±2517 | >10,000 |
| 18 | H | Me | H | Hexanoyl | CH | >10,000 | >10,000 |
| 19 | H | Me | H | 3-Ph-propyl | CH | 3278±639 | >10,000 |
| 20 | H | Me | Me | 3-Ph-propyl | CH | >10,000 | >10,000 |
| 21 | H | Me | H | Hexyl | CH | >10,000 | >10,000 |
| 22 | H | Me | Me | Hexyl | CH | 398±16 | >10,000 |
| 23 | H | H | Me | Me | N | 46.3±8 | >10,000 |
values are the mean of at least three independent experiments in duplicate.
values are the mean of at least two independent experiments in duplicate.
nt = not tested.
