Abstract
The soft zone in dentine beneath the dentino-enamel junction is thought to play an important role in tooth function, strain distribution and fracture resistance during mastication. Recently reported asymmetry in mechanical properties with tooth side may point at a basic property of tooth function. The aim of our study was to test if this asymmetry was reflected in the nano- and micromechanical properties of dentine.
We investigated the mechanical properties of dentine on the buccal and lingual side of nine extracted human teeth using nano- and microindentation. Properties were analysed on the natural log scale, using maximum likelihood to estimate the parameters. Two-sided 0.05-level likelihood ratio tests were used to assess the influences of surface (buccal versus lingual) and dentine depth, measured from the DEJ in crown dentine and from the CDJ in root dentine.
Results showed the well known gradual increase in mechanical properties with increasing distance from the DEJ. Coronal dentine showed higher elastic modulus and hardness on the lingual side of teeth for all measurements, while root dentine was harder on the buccal side. Due to the subtlety of these effects and the small number of teeth studied, results failed to reach statistical significance. Results suggest that dentine nano- and micromechanical properties vary with tooth side in agreement with recent literature using macroscopic methods. They also reveal that buccal-lingual ratios of hardness are in opposite directions in crown and root dentine, suggesting compensatory functions.
Keywords: human teeth, tooth, mantle dentine, dentino-enamel junction, soft dentine, nanoindentation, microhardness, elastic modulus, hardness, atomic-force microscopy
1. Introduction
Dentine is situated between the pulp chamber and enamel (coronal dentine) or cementum (root dentine) of teeth, and its microstructure consists of a hydrated type I collagen matrix reinforced with nanocrystalline carbonated apatite (Ten Cate, 1998). Intertubular dentine (ITD) lies between tubules that run from the pulp chamber to the dentino-enamel junction (DEJ); the tubule lumens are about 1 μm in diameter and are surrounded by a 0.5–1.5 μm hypermineralised layer of peritubular dentine (PTD) (Kinney et al., 1999) which seems to be non-collagenous (Habelitz et al., 2007).
Studies of deformation and stiffness of the dentine soft zone near the DEJ showed dentine near the DEJ to be significantly less stiff on the buccal side than on the lingual side of the tooth, suggesting that tooth function relies on this asymmetry, allowing the enamel cap to shift during mastication (Zaslansky et al., 2006a; Wang and Weiner, 1998). The aim of our preliminary study was to determine if this asymmetry in mechanical properties of teeth was reflected in elastic modulus and hardness of dentine near the DEJ, which we investigated using nano- and microindentation.
2. Materials and methods
Tooth sample preparation
Human maxillary third molars from females aged 19 to 23 years requiring extractions as part of dental treatment were collected following a protocol approved by the UCSF Committee on Human Research. Teeth were sterilised using gamma radiation (White et al., 1994; Brauer et al., 2008) and stored in Hank's Balanced Salt Solution (HBSS) at 4°C. Crowns were sectioned parallel to the occlusal plane to obtain 1–2 mm thick discs from directly beneath the occlusal part of the enamel, using a diamond saw (Buehler, Lake Bluff, IL, USA); roots were sectioned bucco-lingually in the centre of the root, perpendicular to the occlusal plane (for illustration see diagrams in Figure 1). Specimens were polished with SiC papers and diamond suspensions to 0.25 μm and stored in HBSS to minimise surface demineralisation (Habelitz et al., 2002).
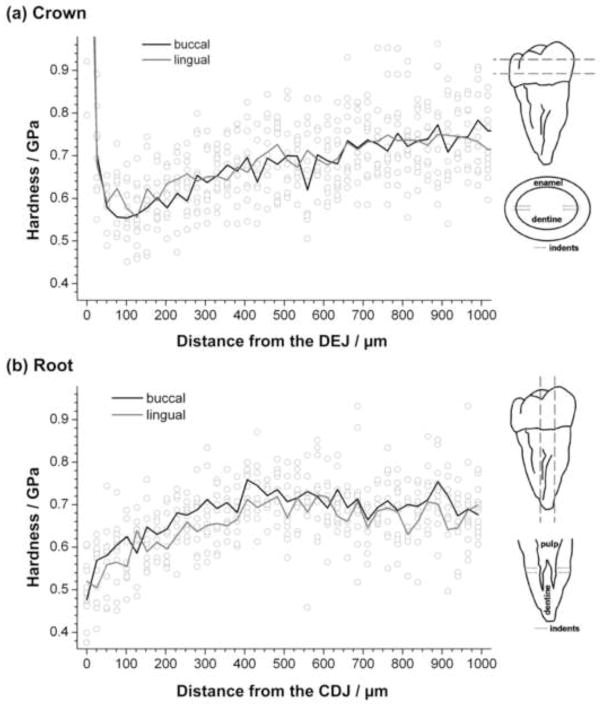
Figure 1.
Results of microindentation experiments on dry (a) crown and (b) root dentine. Hollow circles indicate each single indentation results, while the lines indicate average values from 3 independent tooth samples for buccal (black) and lingual (grey) sides of teeth. Diagrams illustrate sectioning of and indentations on crown (top) and root (bottom) samples.
Materials testing
Three types of experiments were performed: measurement of (i) nano-mechanical properties (elastic modulus and hardness) of wet and dry coronal dentine, (ii) microhardness of coronal dentine and (iii) microhardness of root dentine. For each experiment three independent tooth samples were used, i.e. 9 specimens in total. Nanoindentations were performed using a Nanoscope III (Digital Instruments, Santa Barbara, CA) atomic force microscope (AFM) with the standard head replaced with a TriboScope system and Berkovich indenter (both Hysitron Inc., Minneapolis, MN). Calibration was performed on standard fused quartz samples with known elastic modulus. Peak load of 400 (dentine) and 2000 μN (enamel) produced load-deformation curves, from which both elastic modulus and hardness were calculated as described previously (Brauer et al., 2008); experiments on wet and dry samples were performed on buccal and lingual sides along linear “tracks”, starting in enamel, crossing the DEJ and continuing over > 400 μm into dentine. Step size varied between 1 and 5 μm; smaller step sizes being used near the DEJ.
Microhardness was tested on dry crowns and roots using a microhardness tester (MicroMet 5101, Buehler) with Knoop diamond indenter and loads of 50 (enamel) to 10 g (dentine), using a normal load application with the indenter being in contact with the sample for 10 s. Measurements were started on the DEJ (crowns) or cemento-dentine junction (CDJ; roots) for > 1 mm into dentine (step size 25.4 μm). Measurements on roots were performed halfway between crown (i.e. enamel-cementum junction) and root apex. Knoop hardness (GPa) was determined as:
| (eq. 1) |
where P is the force in N and d is the length of the long diagonal of the indentation (mm).
Both nano- and microindentation measurements were performed along linear “tracks” perpendicular to the junction, with two tracks per tooth side (buccal and lingual) for each sample.
Statistical analysis
Medians and ranges were used to summarise, per track, the total number of indentations and the frequency of indentations per 100 μm of dentine. The large range of distances per track allowed modelling of mean properties (hardness and elastic modulus) by surface (buccal, lingual), distance (linear and quadratic terms), and their interaction. Thus we could examine differences between surfaces near the DEJ and at points (at 50, 100, 200, and 400 μm) between the DEJ and the pulp chamber. The analyses, performed using SAS software v9.1, accounted for correlation among surfaces within teeth via a random effect and for the correlation among measures within tracks using compound symmetry (use of other covariance structures, variance components and autoregressive did not change the results). Properties (hardness and elastic modulus) of dentine were analysed on the natural log scale, using maximum likelihood to estimate parameters and likelihood ratio tests (LRT) to assess variation in dentine properties between surfaces and by distance from the DEJ. Surface effects, estimated as mean buccal-lingual differences and 95% confidence intervals on the log scale, were exponentiated for reporting, yielding buccal-lingual ratio estimates on the natural scale. Both properties were modelled as quadratic functions of distance.
3. Results
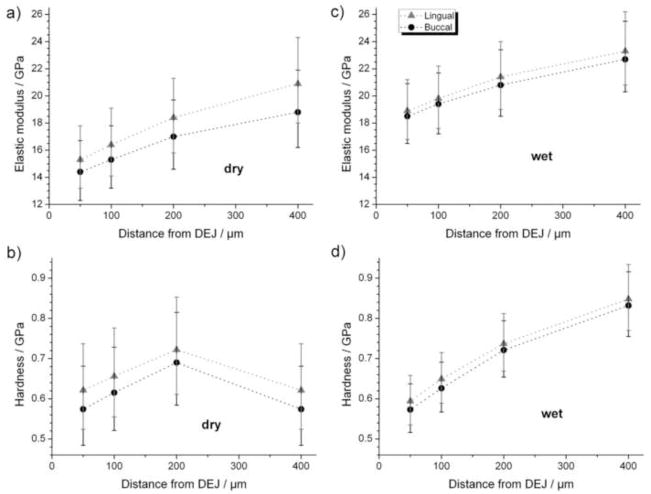
Hardness (Figure 1 for microindentation results) and elastic modulus values showed large variability. At each distance (50, 100, 200 and 400μm) from the DEJ, elastic modulus and hardness from nanoindentation experiments on dry and wet coronal dentine are lower on the buccal than on the lingual side (Figure 2); however, differences are not statistically significant. In addition, elastic modulus and hardness increased with distance from the DEJ but trends were not statistically significant (global LRT, p > 0.05).
Figure 2.
Elastic modulus (top) and hardness (bottom) from nanoindentation experiments of both dry (left) and wet (right) crowns for buccal (black circles) and lingual (grey triangles) sides of teeth as a function of distance from the DEJ. Results are presented as mean ± 95% confidence interval.
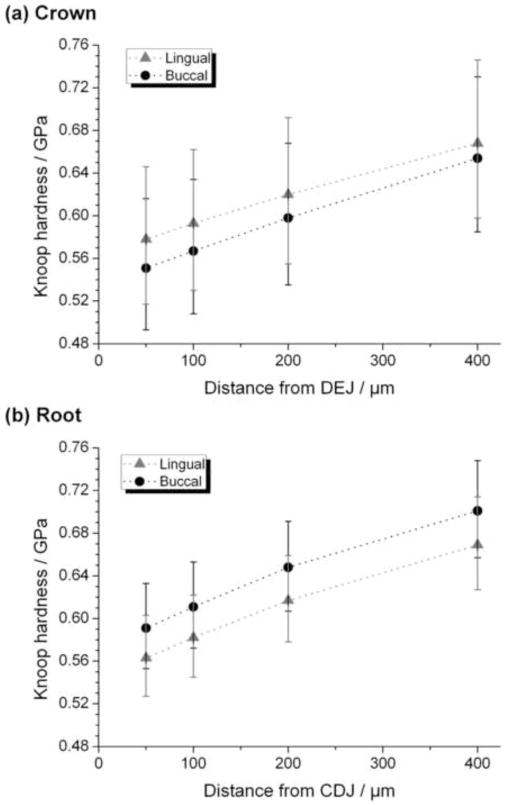
Microhardness of dry coronal dentine (Figure 3a) shows similar trends to those for nanohardness, results being lower on the buccal side and increasing with depth but trends are not statistically significant. Microhardness of dry root dentine (Figure 3b), on the other hand, is higher on buccal sides than on lingual. In root as in crown dentine microhardness increases in deeper dentine; again, results are statistically not significant. On dry coronal dentine nanohardness (Figure 2b) is higher than microhardness (Figure 3a); this is particularly noticeable at 100 to 400 μm from the DEJ.
Figure 3.
Microhardness from microindentation experiments on dry (a) crowns and (b) roots for buccal (black circles) and lingual (grey triangles) sides of teeth as a function of distance from the DEJ (crowns) or CDJ (roots). Results are presented as mean ± 95% confidence interval.
4. Discussion
Dentine is known for its gradual transition in structure and properties (Craig et al., 1959; Wang and Weiner, 1998; Fusayama et al., 1966; Maev et al., 2002; White et al., 2000; Marshall et al., 2001) and this change in mechanical properties is reflected in the observed gradual increase in hardness and elastic modulus with increasing distance from the DEJ. Two main factors can account for this: dentine near the DEJ is known to be softer than bulk dentine, and the number of tubules per unit area, i.e. the fraction of harder and stiffer peritubular dentine, decreases. Due to the large microindenter size in relation to the dentine microstructure, microhardness results give a composite average of peritubular dentine, tubule orifices and intertubular dentine (Kinney et al., 1996) while nanoindentation allows measurement of elastic modulus and hardness in small features, e.g. of peritubular dentine. Therefore nanohardness shows larger maxima due to measurements on peritubular dentine. Nanoindentation also yields larger average hardness than microindentation, particularly at increasing distances from the DEJ, due to microindentations including the empty space of the tubule orifices, giving a smaller average value. This difference between nano- and microindentation measurements becomes more pronounced with increasing number of tubules per unit area at increasing distance from the DEJ.
Recently it was reported that the mechanical properties of the dentine soft zone show a bucco-lingual asymmetry (Zaslansky et al., 2006a) with buccal dentine near the DEJ being less stiff than lingual. The authors suggested that this asymmetry could be related to tooth function during mastication, as the enamel cap was shown to tilt towards the buccal side when loaded (Zaslansky et al., 2006b). Asymmetry in strain and stiffness was reported repeatedly (Wang and Weiner, 1998; Wood et al., 2003; Zaslansky et al., 2006a), and modelling suggested that ITD governed the elastic behaviour of dentine (Kinney et al., 1999).
Our results show buccal coronal dentine to have consistently lower elastic modulus and hardness than lingual, which suggests that the asymmetry found by Zaslansky et al. might at least partially be caused by differences in dentine materials properties with tooth side. Our finding that root dentine on the lingual side is less hard than buccal (opposite to crown dentine) suggests a compensatory effect, through which the root provides stability for the flexibility present in the crown. However, due to the small number of samples used in this study (n = 3 for each method), the differences failed to reach statistical significance at the 0.05 level.
It is worth noting that Zaslansky et al. (2006a) noted the buccal/lingual asymmetry on maxillary premolars, while here also upper teeth were investigated. Considering that in maxillary and mandibular teeth opposite cusps are loaded during mastication, functional differences might be associated with opposing differences in buccal and lingual properties and will be investigated in future experiments.
Despite the limitations of this study due to small sample numbers, our findings provide a potential explanation for the findings of Zaslansky et al.: teeth resist impact forces through asymmetries in mechanical properties between the sides of the tooth, in opposite directions in crown and root dentine.
Acknowledgments
The authors would like to thank Grace Nonomura for collecting the tooth samples and Dr Sunita Ho for use of the microindenter. Support by NIH/NIDCR Grant P01DE09859 is gratefully acknowledged.
Footnotes
Conflicts of interest statement
There are no conflicts of interest to be declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brauer DS, Saeki K, Hilton JF, Marshall GW, Marshall SJ. Effect of sterilization by gamma radiation on nano-mechanical properties of teeth. Dental Materials. 2008;24:1137–1140. doi: 10.1016/j.dental.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Craig RG, Gehring PE, Peyton FA. Relation of structure to the microhardness of human dentin. Journal of Dental Research. 1959;38:624–630. doi: 10.1177/00220345590380032701. [DOI] [PubMed] [Google Scholar]
- Fusayama T, OKUSE K, HOSODA H. Relationship between hardness, discoloration, and microbial invasion in carious dentin. Journal of Dental Research. 1966;45:1033–1046. doi: 10.1177/00220345660450040401. [DOI] [PubMed] [Google Scholar]
- Habelitz S, Marshall GW, Balooch M, Marshall SJ. Nanoindentation and storage of teeth. Journal of Biomechanics. 2002;35:995–998. doi: 10.1016/s0021-9290(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Habelitz S, Rodriguez BJ, Marshall SJ, Marshall GW, Kalinin SV, Gruverman A. Peritubular dentin lacks piezoelectricity. Journal of Dental Research. 2007;86:908–911. doi: 10.1177/154405910708600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JH, Balooch M, Marshall GW, Marshall SJ. A micromechanics model of the elastic properties of human dentine. Archives of Oral Biology. 1999;44:813–822. doi: 10.1016/s0003-9969(99)00080-1. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Balooch M, Marshall SJ, Marshall GW, Weihs TP. Hardness and Young's modulus of human peritubular and intertubular dentine. Archives of Oral Biology. 1996;41:9–13. doi: 10.1016/0003-9969(95)00109-3. [DOI] [PubMed] [Google Scholar]
- Maev RG, Denisova LA, Maeva EY, Denissov AA. New data on histology and physico-mechanical properties of human tooth tissue obtained with acoustic microscopy. Ultrasound in Medicine and Biology. 2002;28:131–136. doi: 10.1016/s0301-5629(01)00480-x. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. Journal of Biomedical Materials Research. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ten Cate AR. Oral Histology: Development, Structure and Function. Mosby; St. Louis, MO: 1998. [Google Scholar]
- Wang RZ, Weiner S. Strain-structure relations in human teeth using Moire fringes. Journal of Biomechanics. 1998;31:135–141. doi: 10.1016/s0021-9290(97)00131-0. [DOI] [PubMed] [Google Scholar]
- White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma-radiation. Journal of Dental Research. 1994;73:1560–1567. doi: 10.1177/00220345940730091201. [DOI] [PubMed] [Google Scholar]
- White SN, Paine ML, Luo W, Sarikaya M, Fong H, Yu ZK, Li ZC, Snead ML. The dentino-enamel junction is a broad transitional zone uniting dissimilar bioceramic composites. Journal of the American Ceramic Society. 2000;83:238–240. [Google Scholar]
- Wood JD, Wang RZ, Weiner S, Pashley DH. Mapping of tooth deformation caused by moisture change using moire interferometry. Dental Materials. 2003;19:159–166. doi: 10.1016/s0109-5641(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Zaslansky P, Friesem AA, Weiner S. Structure and mechanical properties of the soft zone separating bulk dentin and enamel in crowns of human teeth: Insight into tooth function. Journal of Structural Biology. 2006a;153:188–199. doi: 10.1016/j.jsb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Zaslansky P, Shahar R, Friesem AA, Weiner S. Relations between shape, materials properties, and function in biological materials using laser speckle interferometry: In situ tooth deformation. Advanced Functional Materials. 2006b;16:1925–1936. [Google Scholar]