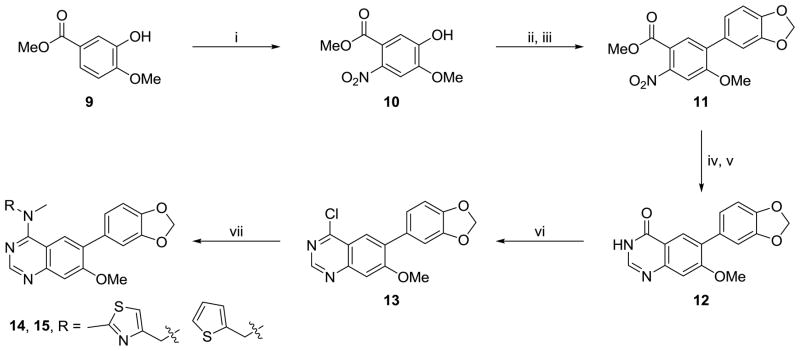
Scheme 2.
Reagents and conditions: (i) HNO3, CH3COOH, 0 °C - r.t., 18 h (ii) triflic anhydride, pyridine, DCM, 1 h, 91% (iii) 3,4-(Methylenedioxy)-phenylboronic acid, Pd(PPh3)4, Na2CO3, DME, H2O, 150 °C (μW), 1 h, 55% (iv) Pd/C, H2 (1 atm), EtOH, 18 h, 99% (v) NH2CHO, NH3COOH, 140 °C for 3 h, then r.t. for 18 h, 51% (vi) POCI3, N,N-dimethylaniline, toluene, reflux, 1 h (vii) RNHCH3, iPr2NEt, DMF, 60 °C, 18 h (typical yields: 80–95%).