Table 2.
Effects of N-arylmethyl substituents on integrase inhibitory potencies of hydroxy-pyrrolopyridine triones (4c) as compared with 4,5-dihydroxy-1H-isoindole-1,3(2H)-diones (3).a
 | ||||||
|---|---|---|---|---|---|---|
| No. | R | IC50 (μM)
|
No. | IC50 (μM)
|
||
| 3′-Processing | Strand Transfer | 3′-Processing | Strand Transfer | |||
| 4c-1 |
|
> 111 | 6.0 ± 0.7 | 3-1 | 14 ± 3 | 0.17 ± 0.06 |
| 4c-2 |
|
> 111 | 10.4 ± 1.0 | 3-2 | 8 ± 3 | 5.3 ± 1.5 |
| 4c-3 |
|
> 111 | 6.01 | 3-3 | 27 ± 1 | 0.14 ± 0.03 |
| 4c-4 |

|
> 111 | 9.2 ± 1.4 | 3-4 | 25 ± 16 | 1.7 ± 0.8 |
| 4c-5 |
|
> 111 | > 111 | 3-5 | 72 ± 23 | 0.39 ± 0.21 |
| 4c-6 |
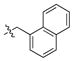
|
> 111 | 90.5 ± 4.7 | 3-6 | 150 ± 36 | 0.72 ± 0.25 |
Data was obtained from in vitro IN assays as described in reference 4.
