Abstract
Cyclin E activates Cdk2, controls centrosome duplication and regulates histone gene transcription. Cyclin E is deregulated in cancer and appears as low molecular weight (LMW) isoforms that correlate strongly with decreased survival in breast cancer patients. Transgenic mice overexpressing LMW cyclin E have increased incidence of mammary tumors and distant metastasis when compared to full length cyclin E. To specifically test the requirement for Cdk2 in LMW-cyclin E mediated mammary tumorigenesis, we generated transgenic mice, which expressed LMW-cyclin E in a Cdk2 deficient background. We found that mammary gland development proceeds relatively normally in these animals, indicating that Cdk2 kinase activity is largely dispensable for this process. However, Cdk2 deficient mice were completely resistant to LMW-cyclin E mediated mammary tumors. Cdk2 wild-type or heterozygous mice succumbed to mammary tumors with mean latencies of 16 and 19.5 months, respectively, but Cdk2 nullizygous littermates did not display tumors through 24 months. Similarly, continuous administration of two different Cdk inhibitors significantly delayed LMW-cyclin E induced mammary tumor progression. Triple transgenic mice generated in a p53 heterozygous background also displayed no tumors. We also found that Cdk2 silencing induced cell death in LMW-overexpressing breast cancer cell lines, but not in cell lines lacking LMW expression. Our findings establish a requirement for Cdk2 in LMW-cyclin E mediated mammary tumorigenesis, arguing that human breast tumors overexpressing LMW-cyclin E are prime candidates for anti-Cdk2 therapy.
Keywords: Low molecular weight cyclin E, Cdk2, transgenic mice, breast cancer, roscovitine, meriolin
Introduction
Progression through the cell cycle is driven by cyclin-dependent kinases (Cdks) whose catalytic activity and substrate specificity depend on their association with regulatory subunits called cyclins. Altered expression of cyclins can drive aberrant proliferation in cancer (1). Cyclin E is of particular importance, as its expression is deregulated in many cancers, most notably breast cancer (2-4). One mode of deregulation of cyclin E expression is the generation of low-molecular-weight (LMW) isoforms following cleavage of full length cyclin E by an elastase like protease (5). A consequence for cells with LMW cyclin E expression is increased genomic instability (6) due to premature activation of CDC25C (7) and shortening of the length of mitosis from nuclear envelope breakdown to prometaphase (8). Clinically, the expression of LMW forms of cyclin E correlates strongly with decreased survival in patients with breast cancer (9). We have also examined the oncogenic potential of these isoforms and show that transgenic mice overexpressing LMW cyclin E had increased incidence of mammary tumors and distant metastasis when compared to full length cyclin E (10). The critical role of LMW cyclin E in mammary tumorigenesis may be the result of the ability of LMW cyclin E to bind and activate its cyclin-dependent kinase partner, Cdk2. These isoforms bind more tightly to Cdk2, which leads to increased Cdk2 kinase activity and decreased sensitivity of the Cdk2 complexes to inhibition by p21 and p27 (6, 11). The LMW cyclin E expressing cells are also resistant to anti-estrogens (6) and to aromatase inhibitors (12) due to decreased sensitivity of the LMW cyclin E/Cdk2 complexes to p21 and p27, which are induced in response to anti-estrogen treatment.
Cdk2 is a critical enzyme in the transition of cells from G1 to S phase and its deregulation in cancer could be causative of oncogenesis. One of the functions of Cdk2 (in complex with either cyclin E or cyclin A) is to phosphorylate substrates such as the retinoblastoma protein (pRB) that activates the genes necessary for S phase through E2F-dependent transcription. Additional substrates of cyclin E/Cdk2 complexes include NPAT, a transcription factor that controls cell cycle dependent histone gene transcription (13), nucleophosmin (14), CP110 (15), and Mps1 (16), proteins involved in centrosome duplication, Brca1 (17) and Ku70 (18), involve in DNA repair and Cdk inhibitors, p21 and p27. However, a number of kinase-independent functions of cyclin E have been described including a role in replication endocycle (19, 20), in replication licensing during exit from quiescence (21), in oncogenic transformation by ras and dominant negative p53 (21) and in cell fate determination (22).
Cdk2 independent functions of cyclin E are also inferred from in vivo models. For example, knockout of both cyclin E1 and cyclin E2 genes in the mouse leads to embryonic lethality due to defects in the endo-reduplication of trophoblast cells (19, 20) while Cdk2 knockout mice do not have placental defects suggesting that E-type cyclins have Cdk2 independent roles in early development (21). A kinase-deficient cyclin E mutant can partially restore MCM loading and exit from quiescence in cyclin E-null cells (21). Cyclin E also localizes to the centrosomes in a Cdk2 independent fashion and mutation of the centrosomal localization sequence prevents the mutated overexpressed cyclin E to increase the S-phase fraction of cells (23). In drosophila, even when p21 is overexpressed, cyclin E is required for the initial asymmetric division of certain neuroblasts for neural segment-specific versus abdomen lineages suggesting a role in cell fate determination (22). These kinase-independent functions of the overexpressed cyclin E might facilitate the escape of tumor cells from quiescence, and might modify their cell fate, thereby contributing to cancer formation. A major, unresolved issue is the contribution of these various kinase-dependent and kinase-independent functions of LMW cyclin E to tumorigenesis. In this study we have addressed the question of the requirement of Cdk2 for LMW cyclin E-induced mammary tumorigenesis function by generating LMW cyclin E transgenic mice with either a Cdk2 wild-type or knock out background. These studies show that LMW-cyclin E mediated tumorigenesis is completely dependent on the presence of Cdk2.
Materials and Methods
Generation and analysis of transgenic mice
Generation of Cdk2-deficient mice (24), p53-deficent mice (25) and MMTV-LMW cyclin E transgenic mice (10) were described previously. As Cdk2-/- mice were reported to be sterile, the mice strain was maintained as Cdk2+/- mice and genotyped as previously described (24). Since all our transgenic lines were created and maintained on an inbred FVB/N background, Cdk2+/- mice created on a 129/CD-1 background were backcrossed seven generations into an FVB/N background prior to crosses with p53+/- and LMW cyclin E transgenic lines. Mating MMTV-LMW cyclin E; Cdk2+/- mice with Cdk2+/- mice yielded the 3 experimental groups MMTV-LMW cyclin E; Cdk2+/+, MMTV-LMW cyclin E; Cdk2+/-, and MMTV-LMW cyclin E; Cdk2-/-. Crossing MMTV-LMW cyclin E; Cdk2+/- mice with p53-/-;Cdk2+/- mice yielded the 3 experimental groups MMTV-LMW cyclin E; p53+/-; Cdk2+/+, MMTV-LMW cyclin E; p53+/-; Cdk2+/-, and MMTV-LMW cyclin E; p53+/-; Cdk2-/-. Mice were kept as virgins and were monitored biweekly, by palpation, for tumor occurrence. Animals displaying tumors (with a mean diameter of no more than1.5 centimeters) were sacrificed, and the presence of mammary adenocarcinomas was confirmed histologically. All experiments were approved by the Animal Care and Use Committee at the University of Texas, MD Anderson Cancer Center and were performed in accordance with relevant institutional and national guidelines and regulations.
Roscovitine and meriolin 5 treatment of mice
Roscovitine and meriolin 5 were supplied by Dr Laurent Meijer (Roscoff, France). A Roscovitine stock solution of 10 mg/ml (28 mM) in 50 mM HCl pH 2.5 was prepared and stored at -20C. Mice were randomly separated in the control and test groups, which were injected i.p. with 50 mM HCl pH2.5 or Roscovitine (75 mg/kg) respectively at 10 ul of stock solution/g of mouse weight i.p. 2 times daily for 7 days. A meriolin 5 stock solution of 3.333 mg/mL was prepared in DMSO. For daily injections of laboratory mice, a working solution of 0.1 mg/mL was freshly prepared with sterile PBS (30:70 v/v). Mice were randomly separated in the control and test groups, which were injected i.p. with DMSO/PBS (30:70 v/v) or meriolin 5 (1 mg/kg per day), for two series of 5 days with a 2-day break in between. Statistical significance was assessed using the log-rank test of Kaplan-Meier analysis.
Hormone treatment of mice
Twelve-week old, nulliparous mice were treated for 48 h with a single interscapular subcutaneous injection of 17β-estradiol benzoate (1 μg) and progesterone (1 mg) in 100 μl of sesame oil (all from Sigma). Two hours before sacrifice, animals were injected intraperitoneally with BrdUrd (0.03 mg/g of body weight; Sigma). Mammary glands were removed and fixed in 10% formalin overnight. After embedding in paraffin, tissues were sectioned (5–7 μm) onto Probe-On Plus charged slides (Fisher Scientific).
Whole-mount mammary gland staining, and histology
Mammary glands from selected mice were dissected out and fixed on glass slides with Carnoy's solution (glacial acetic : choloroform : ethanol, 1 : 3 : 6) overnight at room temperature. The glands were rehydrated prior to overnight staining in aluminum carmine (1 g carmine, 2.5 g aluminum potassium sulfate boiled for 20 min in distilled H20, filtered and brought to a final volume of 500 ml). The glands were then dehydrated, cleared with xylene and mounted. Photographs were taken under 4X-power objective using a digital camera mounted on a Leica MZ125 microscope. For histological analysis, 6 mm sections were cut and stained with Hematoxylin and Eosin.
5-bromo-2-deoxyuridine incorporation assay
To assess cell proliferation, mice were injected i.p. with 0.25 mg 5-bromo-2-deoxyuridine (BrdUrd)/g of body weight 2 h before sacrifice. BrdUrd incorporation was detected on sections by immunohistochemistry using a cell proliferation kit (Amersham) following the manufacturer's instructions. The numbers of BrdUrd positive cells in wild-type and MMTV-cyclin E mammary glands were counted in 10 fields under a 40× objective lens.
Western blot analysis, immunoprecipitation, kinase assays, and immunohistochemistry
Cell lysates were prepared and subjected to western blot analysis as previously described (26). Briefly, 50 μg of protein was subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to Immobilon P overnight at 4°C at 35 mV constant voltage. The blots were blocked overnight at 4°C in BLOTTO (5% nonfat dried milk in 20 mM Tris, 137 mM NaCl, 0.05% Tween, pH 7.6). After being washed, the blots were incubated in primary antibodies for 3 hr. Primary antibodies used were cyclin E (HE-12; Santa Cruz Biotechnology), Cdk2 (Transduction Laboratories), and actin (Chemicon International Inc., Temecula, CA). Blots were incubated with goat anti-mouse immunoglobulin-horseradish peroxidase conjugate at a dilution of 1:5,000 in BLOTTO for 1 hr and finally washed and developed by using the Renaissance chemiluminescence system as directed by the manufacturer (Perkin Elmer Life Sciences, Inc., Boston, MA). For immunoprecipitation (IP), two hundred fifty micrograms of tissue extracts were used per immunoprecipitation with polyclonal antibody to cyclin E coupled to protein A beads. After being washed, the immunoprecipitates were incubated with kinase assay buffer containing 60 mM cold ATP, 5 mCi of [32P]ATP, and 5 mg of histone H1 (Roche Diagnostics Corporation, Indianapolis, IN) in a final volume of 30 μl at 37°C for 30 min. The products of the reaction were analyzed on 13% SDS-PAGE gels, and the gels were stained, destained, dried, and exposed to X-ray film. For quantitation, the protein bands corresponding to histone H1 were excised, and the radioactivity of each band was measured by Cerenkov counting. For immunohistochemistry, the sections were incubated in 1% H2O2 to block endogenous peroxidase activity. To retrieve nuclear antigens on paraffin embedded sections, slides were incubated for 20 min in 10 mmol/L sodium citrate buffer (pH 6.0) at 90 C. The sections were then incubated for 60 min in 5% FCS overnight with primary antibodies, followed by 2-h incubation at room temperature with appropriate secondary antibodies. Nuclei were counterstained with hematoxylin. Rabbit polyclonal anti–cyclin E (Santa Cruz Biotechnology, sc-198 (C19) that specifically recognized the human cyclin E protein was used for the transgenic cyclin E. For detection, the Vectastain ABC Elite kit (Vector Labs) was used.
Compounds, Cell lines, and culture conditions
CVT-313, a selective and potent inhibitor of Cdk2, was obtained from Enzo Life Sciences International while roscovitine and meriolin 5 were provided by Dr Laurent Meijer (Roscoff, France). Serum was purchased from Hyclone Laboratories (Logan, Utah, USA) and cell culture medium from Life Technologies, Inc. (Grand Island, NY, USA). The culture conditions for HCC1806, HCC1569, ZR75-1, UACC812 and MCF-7 breast cancer cell lines were described previously (27, 28). All cell lines were purchased from ATCC and used within 6 months.
RNA interference
The synthetic siRNA oligonucleotides were synthetized by Sigma. siRNAs were targeting Cdk2 on nucleotides 274-292 in NM_001798 for Cdk2 siRNA#1 (ID# SASI_Hs01_00060175) and on nucleotides 308-326 in NM_001798 for Cdk2 siRNA#2 (ID# SASI_Hs01_00060174). A MISSION siRNA Universal Negative Control #1 was used as negative control (Neg). Breast cancer cell lines were cultured for 24 hours in 6-well plates and transfected with 2 μg siRNA using X-tremeGENE siRNA Transfection Reagent (Roche) according to manufacturer's protocol. For MCF-7 LMW cyclin E (T2) cells, doxycycline (1 μg/ml) was used to induce expression of LMW (T2) cyclin E for 24 hours before siRNA transfection.
MTT metabolization Assay
Cell proliferation assays were carried out using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, where MTT is reduced to purple formazan by the mitochondria of living cells. Increase in cell number is detected by augmented MTT metabolization, and decrease in cell number is reflected by decrease in MTT metabolization. MCF-7 cells that can inducibly express Flag-tagged LMW Cyclin E (T2) upon treatment with doxycycline (described in (7)) were plated at a density of 2,500 cells per well in 96-well plates and cultured overnight in MEM supplemented with 10% FBS. Cells were incubated with or without doxycycline (Dox, 1 ug/mL) for 24 h, and cells were then treated with either diluent (DMSO) or CVT-313 (29), or meriolin 5 (30), or roscovitine (31) at 8 different concentrations for 48 hrs. Stock solution for each drug was made in DMSO at a concentration of 25 mM for CVT-313, 10 mM for roscovitine, and 1 mM for meriolin 5 followed by 2-fold serial dilution in the medium. Each well was replaced with 200 uL of fresh medium containing MTT (0.5 ug/uL) and incubated for 4 hours. The medium was then removed and 100 uL of solubilization buffer (0.04 M HCl, 1% SDS in isopropyl alcohol) was added to each well. The plate was agitated in the dark for 5 minutes to dissolve the MTT-formazan crystals. The absorbance of the samples was recorded at 590 nm in a multiwell plate reader (Perkin Elmer Victor 1420). Results were plotted as the mean (95% CI) values of quadruplicates from a representative experiment that was repeated at least two independent times.
Statistical analysis
Tumor onset data were analyzed for statistical significance by using survival analysis methods. Within each genotype, tumor-free survival curves were estimated by the Kaplan-Meier method and compared by using the log-rank test using Prism (GraphPad software, Inc.). Results are shown as mean ± SD. Differences were considered significant when the two-tailed Student's t test showed differences at P < 0.05.
Results
Decreased branching and proliferation in Cdk2-/- mammary glands can be rescued by ovarian hormone treatment
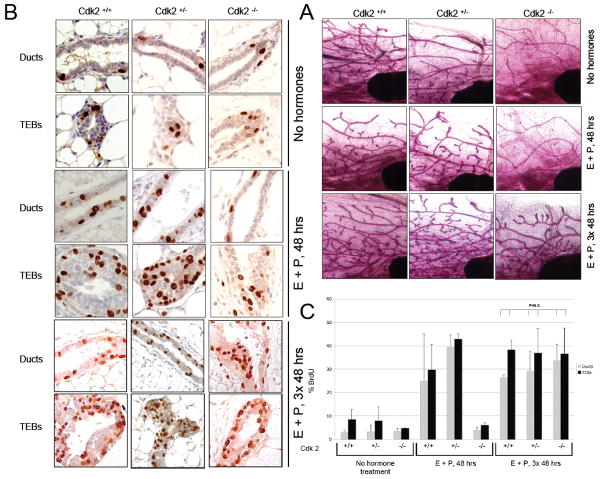
We compared the appearance of mammary glands of adult, virgin Cdk2-/- females with that of Cdk2+/+ and Cdk2+/- by whole mounts stained with carmine red (Fig 1A). The results reveal that while the development of the whole mammary glands proceeds relatively normally in all three genotypes, that the branching morphogenesis is decreased in the Cdk2-/- animals (Figure 1A). This raised the question if there is a defect in the ability of the mammary cells to proliferate in the Cdk2-/- mice, or if the decreased branching is linked to an indirect effect of Cdk2 loss on the synthesis of ovarian hormones due to the reduced size of the ovaries. To address this question we examined the ability of mammary epithelial cells in each of the three genotypes to proliferate in response to pregnancy hormones, estrogen and progesterone. To mimic the burst of proliferation observed during early pregnancy, Cdk2 +/+, Cdk2 +/- and Cdk2 -/- mice were treated acutely with 1 μg of estrogen and 1 mg of progesterone either once for 48 hours or 3 times, 48 hours apart. The 2 hormones were injected in 100 μl of sesame oil under the skin between the shoulder blades. Two hours before sacrifice, the mice were injected with BrdUrd intraperitoneally. The mammary glands were removed and used for whole mounts (Fig. 1A) and BrdUrd staining (Fig. 1B) with quantification in Fig 1C. In the absence of hormones, the whole mount of Cdk2-/- mammary glands showed normal duct formation but decreased branching with a 2-fold decrease in proliferation in the terminal end buds (TEBs) (4.8% versus 8.5% of BrdUrd positive cells in Cdk2+/+). Cdk2-/- mice treated once for 48 hours with E+P showed a slight increase in proliferation in the TEBs with 6.1% of BrdUrd positive cells but well below the 30-40% of BrdUrd positive cells in Cdk2+/+ and Cdk2+/- mammary glands. However, when mice were treated with hormones for 3 times 48 hours apart, then 35 to 40% of mammary cells were BrdUrd positive in Cdk2-/- mammary glands, a percentage similar to Cdk2+/+ and +/- mammary glands (Fig. 1B, C). Collectively, these results suggest that the absence of Cdk2 delays proliferation of the mammary cells in the terminal end buds, however this delay can be rescued by sustained treatment with estrogen and progesterone. These results also suggest that the absence of Cdk2 has not altered the ability of mammary epithelial cells to proliferate in response to stimuli, in this case pregnancy hormones.
Figure 1. Hormone induced proliferation in Cdk2-/- mammary glands.
A. A cohort of mice were left untreated (No hormones) or treated acutely (48–50 h) with estradiol benzoate (E, 1 μg, Sigma, St. Louis, MO) and P (1 mg, Sigma) in 100 μl sesame oil via a single interscapular subcutaneous injection behind the neck once (E+P, 48 hrs) or 3 times, 48 hours apart (E+P, 3× 48 hrs). Two hours before sacrifice, the mice were injected with BrdUrd (0.03 mg/g of body weight) intraperitoneally. Following treatment, the contralateral inguinal gland was removed (Cdk2+/+ mice, n = 3; Cdk2+/- mice, n = 3; Cdk2-/- mice, n = 3, 11–32 weeks of age at day 0 of experiment) and whole-mount were prepared. B. and C. Proliferation is quantified by BrdU staining.
Resistance of Cdk2-/- mice to LMW cyclin E-induced breast cancer
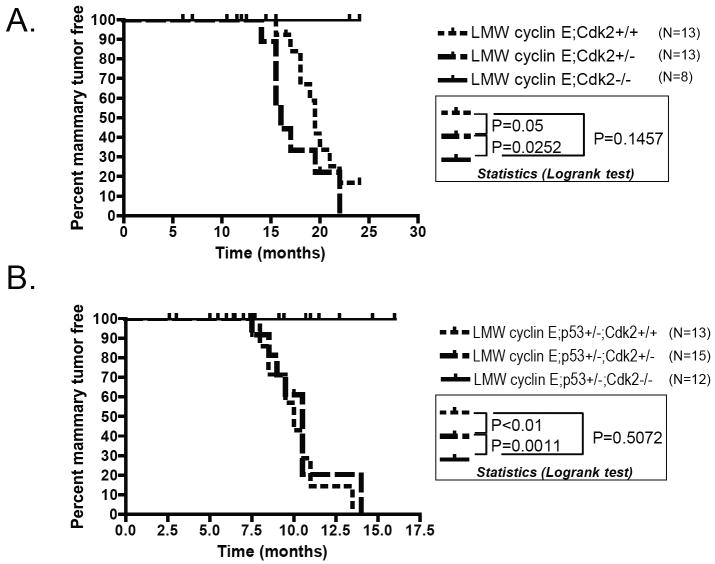
The critical role of LMW cyclin E in mammary oncogenesis and tumorigenesis (6, 32) may be the result of the ability of LMW cyclin E to bind and activate Cdk2. To specifically test the importance of LMW cyclin E associated Cdk2 kinase activity in mammary oncogenesis, we generated double and triple transgenic mice with the genotypes LMW cyclin E /Cdk2 -/- and LMW cyclin E/p53+/-/Cdk2-/-. The transgene in all these animals is under the control of the MMTV promoter. We followed the MMTV-LMW cyclin E mice in the different genotypes for mammary tumor incidence and found that the loss of Cdk2 protected Cdk2-/- mice from breast cancer induced by LMW cyclin E in the p53+/+ (Fig 2A) or p53+/- (Fig 2B) background. Whereas LMW cyclin E mice in Cdk2+/- or Cdk2+/+ background succumb to mammary tumors with mean latencies of 16 and 19.5 months respectively, their LMW cyclin E; Cdk2-/- littermates do not form mammary tumors (Fig 2A). Similarly, while MMTV-LMW-cyclin E/p53+/- transgenic mice in Cdk2+/- or Cdk2+/+ background succumb to mammary gland tumors between 7 and 13 months with 100% penetrance, their MMTV-LMW cyclin E ;p53+/-; Cdk2-/- littermates are resistant to tumor formation (Fig 2B). These results clearly show that Cdk2 is critically required for LMW cyclin E induced mammary tumorigenesis and consequently, the loss of Cdk2 renders Cdk2-/- mice resistant to breast cancer induced by LMW cyclin E expression.
Figure 2. Resistance of Cdk2-/- mice to LMW cyclin E-induced breast cancer.
Percentage of mammary tumor free mice among MMTV-LMW cyclin E-T1; Cdk2+/+; MMTV-LMW cyclin E-T1; Cdk2+/- ; MMTV-LMW cyclin E-T1; Cdk2-/- females in A. and MMTV-LMW cyclin E-T1; p53+/-; Cdk2+/+; MMTVLMW cyclin E-T1; p53+/-; Cdk2+/-; MMTV-LMW cyclin E-T1; p53+/-; Cdk2-/- females in B. Whereas LMW-cyclin E-T1 mice in Cdk2+/- or Cdk2+/+ background succomb to mammary tumors with mean latencies of 16 and 19.5 months respectively, their LMW cyclin E-T1; Cdk2-/- littermates do not form mammary tumors (P=0.05 Cdk2+/+ versus Cdk2-/-; P=0.0252 Cdk2+/- versus Cdk2-/-; P=0.1457 Cdk2+/+ versus Cdk2+/-, Logrank test). Similarly, MMTV-LMW cyclin E T1/p53+/- transgenic mice in Cdk2+/- or Cdk2+/+ background succomb to mammary gland tumors between 7 and 13 months with 100% penetrance, their MMTV-LMW cyclin E-T1;p53+/-; Cdk2-/- littermates are resistant to tumor formation (P<0.01 Cdk2+/+ versus CDk2-/-; P=0.011 Cdk2+/- versus Cdk2-/-, P=0.5072 Cdk2+/+ versus Cdk2-/-, Logrank Test).
Cdk2 absence does not prevent LMW cyclin E expression in the mammary gland
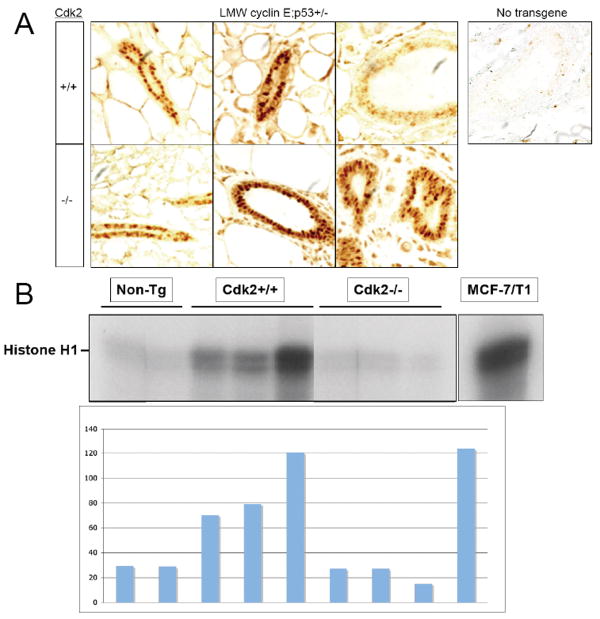
Next, we examined if in the absence of Cdk2 the cyclin E transgene is expressed equally in each genotype. To this end we examined cyclin E levels by immunohistochemical analysis in the mammary glands of LMW cyclin E transgenic mice in a Cdk2+/+ or Cdk 2-/- background (Fig 3A). The results revealed that similar levels of the transgene expression in the mammary glands of Cdk2+/+; and Cdk2-/- females were observed. Additionally, the ducts in each genotype had similarly normal morphology (Fig 3A). These results rule out the possibility that the differences in breast cancer free survival could be caused by an inadequate expression of LMW cyclin E transgene in the mammary glands of Cdk2-/- animals and that Cdk2 absence does not prevent LMW cyclin E expression in the mammary glands.
Figure 3. Cdk2 absence does not prevent LMW cyclin E expression in the mammary gland.
(A) Immunohistochemistry for human cyclin E protein in mammary glands of MMTV-LMW cyclin E-T1; p53+/-; Cdk2+/+ and MMTV-LMW cyclin E-T1; p53+/-; Cdk2-/-. (B) Cyclin E-associated kinase assays of mammary glands protein lysates from age-matched wild-type (Non-Tg), MMTV-LMW cyclin E; p53+/- mice in Cdk2+/+, Cdk2+/- or Cdk2-/- background was carried out with a cyclin E antibody (HE12) using histone H1 as substrate. The histogram show the relative activities after densitometric scanning (arbitrary units).
We then asked if the cyclin E associated kinase activity in the Cdk2-/- mammary glands was completely dependent on Cdk2. We measured the cyclin E associated kinase activity using histone H1 and found a total absence of cyclin E associated kinase activity in the Cdk2-/- mammary glands (Fig. 3B). Hence, even though the LMW cyclin E is being expressed in the mammary gland (Fig 3A) the protein does not retain any cyclin E associated kinase activity in a Cdk2 -/- background.
Roscovitine and meriolin treatment delay LMW cyclin E-induced breast cancer
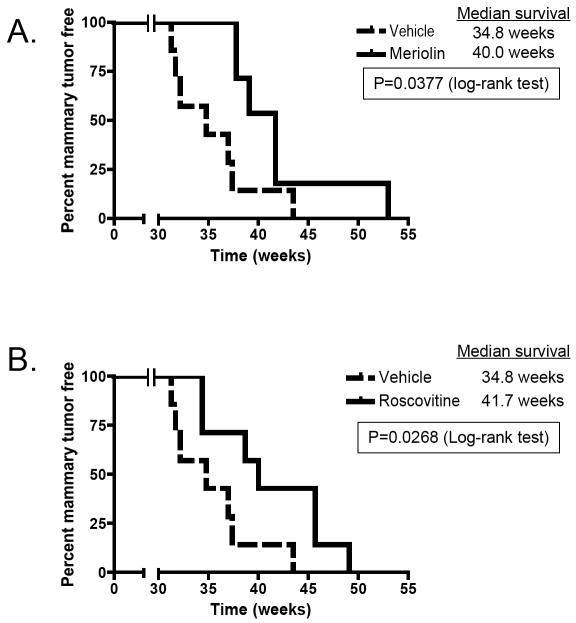
Next, we set out to investigate if targeting cyclin dependent kinase using roscovitine and meriolin 5, two Cdk2 inhibitors, would suppress mammary tumorigenesis in MMTV- LMW cyclin E transgenic mice in a Cdk2+/+ background. Roscovitine (31) is a potent and selective inhibitor of Cdk1, Cdk2, and Cdk5, with strong antiproliferative and apoptotic effects in preclinical models and antitumor activity in several xenograft models. (R)-Roscovitine is well tolerated when administered by intraperitoneal injection, and displays high bioavailability (33). To assess if treatment of the LMW cyclin E transgenic mice by roscovitine would delay tumor formation, we intraperitoneally injected a group of 7 mice with DMSO and a group of 7 mice with roscovitine starting at 7 months, twice a day for 7 days. We used the LMW cyclin E;p53+/- double transgenic animals for these studies as tumor formation in these animals occurs early at 7 months and with 100% penetrance. Treatment of these mice with roscovitine resulted in a significant delay in tumor formation. Specifically, we found a 5.2-week difference in improved median survival for roscovitine-treated mice compared to DMSO-treated mice (Fig. 4A). Similar tumor delay was observed when a novel analogue of roscovitine, meriolin 5 was used. Meriolin 5 belongs to a new family of inhibitors of cyclin dependent kinases with enhanced selectivity with marked potency for Cdk2 and Cdk9, binding within the ATP binding site of the kinase. Meriolin 5 displays antiproliferative and proapoptotic properties in human tumor cell cultures (30, 34). For these experiments a group of 7 LMW cyclin E;p53+/- double transgenic mice were injected with meriolin 5, single daily intraperitoneal injections at a dose of 1 mg/kg for two series of 5 days with a 2-day break in between (Fig 4B). We found a 6.9 weeks difference in median survival for meriolin 5-treated mice compared to DMSO-treated (drug carrier) mice. These results indicate that these Cdk cell cycle inhibitors may have some therapeutic activity in LMW cyclin E overexpressing tumors as shown in this preclinical model.
Figure 4. Roscovitine and meriolin treatment delay LMW cyclin E-induced breast cancer.
A: Tumor-free survival after roscovitine treatment. Randomly selected MMTV-LMW cyclin E-T1; p53+/- mice were treated either with DMSO (n=7) or with roscovitine (75mg/kg, twice daily, n=7) for 1 week. B. Randomly selected MMTV-LMW cyclin E-T1; p53+/- mice were treated either with DMSO (n=7) or with meriolin 5 (1mg/kg, for two series of 5 days with a 2-day break in between, n=7).
Silencing of Cdk2 is lethal to LMW expressing breast cancer cell lines
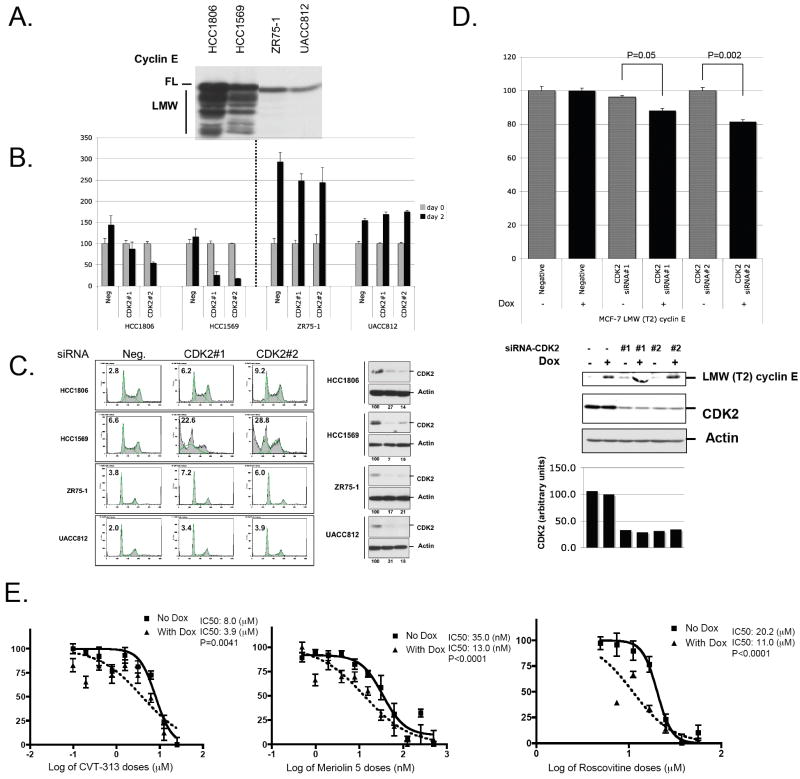
To evaluate Cdk2 as a potential drug target, we silenced Cdk2 expression by siRNA in 2 breast cancer cell lines expressing LMW cyclin E, HCC1806 and HCC1569, and in 2 breast cancer cell lines with no LMW cyclin E expression, ZR75-1 and UACC812 (Fig. 5A). Two days following Cdk2 siRNA transfection, all the cell lines transfected with the scrambled siRNA increased their cell number by 1.44-; 1.16-, 2.93- and 1.55-fold for HCC1806, HCC1569, ZR-75-1 and UACC812 respectively (Fig. 5B). Cdk2 siRNA transfection did not affect the growth of the 2 cell lines with no LMW expression while Cdk2 knock down in LMW expressing line HCC1806 and 1569 led to a strong diminution in cell number so that at 2 days following transfection only 50% of plated cells for 1806 and 10% of plated cells for 1569 remained on each plate (Fig. 5B). We next asked if the decrease in cell number following Cdk2 knock down is due to cell cycle arrest or due to cell death. Propidium iodide staining followed by FACS demonstrated that Cdk2 knock down in LMW cyclin E expressing line HCC1569 and 1806 led to a strong induction of apoptosis with a 3 to 4-fold increase in the percentage of cells in sub-G1 compared to cells transfected with the negative control siRNA. In contrast, in the low LMW cyclin E lines, there is less than a 2-fold increase in the percentage of cells in sub-G1. These results suggest that Cdk2 knock-down causes apoptosis/cell death in LMW cyclin E expressing breast cancer cell lines (Fig. 5C).
Figure 5. Cdk2 inhibition causes apoptosis and inhibition of cell growth in LMW cyclin E overexpressing breast cancer cell lines.
A. Two breast cancer cell lines expressing LMW cyclin E, HCC1806 and HCC1569, and 2 breast cancer cell lines with no LMW cyclin E expression, ZR75-1 and UACC812 were harvested at 48 hours and immunoblotted for cyclin E. B. 100,000 cells for each cell lines were plated at day 0, transfected with scrambled siRNA (Neg) or siRNA against Cdk2 (#1 and #2). Samples were harvested at 48 hrs and counted using a coulter counter. C. FACS analysis of the same cells as in B. (left panel) and western blot analysis for Cdk2 and actin (rigth panel). The levels of proteins were measured by densitometric scanning of the corresponding bands and normalized using actin values. The values indicated at the bottom (in %) were compared with the values obtained with scrambled siRNA transfected cells set at 100%. D. pTRE-LMW Cyclin E (T2) stably transfected MCF-7 cells were incubated with or without doxycycline (Dox, 1 ⌠g/mL) for 24 h then transfected with 21-bp double strand siRNA against Cdk2 or with a negative control siRNA. Cells were collected 48 hours later and used for counting or western blot analysis with cyclin E (T2), Cdk2 and actin antibodies. E. Effect of the expression of LMW Cyclin E (T2) induced by doxycycline in MCF-7 cells on cyclin-dependent kinase inhibitors response. pTRE-LMW Cyclin E–transfected MCF-7 cells (2,500 cells per well) were incubated in 96-well plates for 24 hrs, incubated with or without doxycycline (Dox, 1 ⌠g/mL) for 24 h, and cells were then treated with either diluent (DMSO) or CVT-313, or meriolin 5, or roscovitine at 8 different concentrations for 48 hrs and 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetraolium bromide (MTT) metabolization was measured. Absorbance was measured at 590 nm. The mean absorbance values of diluent-treated samples were taken as 100%. Absorbance values of the cells treated with different drugs are plotted as percentages with respect to the mean value of the diluent-treated samples. A least-squares fit was obtained to estimate the IC50. Means and 95% confidence intervals of an experiment representative of three independent experiments performed in quadruplicate are shown.
Next, we asked if cell death observed following Cdk2 inhibition in breast cancer cells is dependent on LMW cyclin E expression. For these experiments we used MCF-7 cells that can inducibly express Flag-tagged LMW cyclin E (T2) upon treatment with doxycycline (7). LMW cyclin E (T2) was induced for 24 hours followed by silencing of Cdk2 expression by transient siRNA transfection (Fig. 5D). Cdk2 downregulation resulted in selective inhibition of cell growth at 48 hrs when LMW cyclin E is induced. Cell proliferation is reduced by 11.5 % for Cdk2 siRNA#1 and by 18.5 % for siRNA#2, p=0.05 and P=0.002, compared to control siRNA transfected cells only when LMW cyclin E is induced. Down regulation of Cdk2 had no affect on cell proliferation when LMW cyclin E is not induced (Fig. 5D). To extend these findings to clinically applicable compounds, we used 3 different cyclin-dependent kinase inhibitors (CVT-313, meriolin 5, and roscovitine) at different concentrations and compared the sensitivity of MCF-7 cells in the LMW cyclin E on (With Dox) and off (No Dox) settings. CVT-313 is a potent Cdk2 inhibitor, which was identified from a purine analog library and was shown to arrest human cells in G1 (29). For each of the 3 drugs, the IC50 was reduced by at least 2-fold when LMW cyclin E was expressed (Fig. 5E). These findings show that cell growth inhibition following cyclin-dependent kinase inhibition is dependent on LMW cyclin E overexpression.
Discussion
Previously we showed that expression of LMW cyclin E under the control of a mouse mammary tumor virus (MMTV) promoter results in the development of mammary tumors in 27 % of the transgenic mice with a latency of 17-19 months and a third of these mice develop lung metastasis. When MMTV-LMW cyclin E mice are crossed with p53+/- mice, 100% of double transgenic mice develop mammary tumors with a latency of 11 months (10). In our cohort of FVB strain of mice, the p53+/- allele does not predispose to p53-mediated mammary tumor formation. Expression of LMW cyclin E in breast cancer cells induces genomic instability (6-8), and resistance of Cdk2 complexes to inhibition by p21 and p27 (32). Our results, presented in this paper show that Cdk2 is essential for oncogenic LMW cyclin E-induced breast tumorigenesis. Consistent with this finding, inhibition of cyclin-dependent kinase activities using roscovitine or meriolin delay LMW-cyclin E-induced breast cancer in this mouse model. Furthermore, targeted silencing of Cdk2 induced cell death in LMW overexpressing breast cancer cell lines, but not in cell lines with no LMW expression. Consequently, mammary epithelial cells expressing LMW cyclin E are unable to initiate transformation in the absence of Cdk2 while the oncogenic LMW cyclin E/Cdk2 kinase provide breast cancer cells with the capabilities to resist cell death and as such is required to sustain tumor cell proliferation.
We also show that Cdk2 development of the mammary glands and ducts proceed relatively normally except for a slight decrease in branching in the Cdk2-/- animals with a concomitant 2-fold decrease in the proliferation of the terminal end buds. When stimulated by ovarian hormones, the absence of Cdk2 delays proliferation of the mammary cells but this delay can be rescued by sustained treatment with estrogen and progesterone. This rescue experiment shows that Cdk2 is not required for developmental proliferation but the delayed proliferative response of Cdk2-/- mammary cells may be linked to the reduced size of the ovaries of these mice. Other studies showed that Cdk2 is not required for proliferation and differentiation of hematopoietic cells in vivo (35) nor is it required for neural progenitor cells proliferation, differentiation and survival of hippocampal granule neurons in vivo (36). In the adult subventricular zone, it was shown that Cdk2 is critical for proliferation and self-renewal of neural progenitor cells when Cdk4 expression is too low to compensate for loss of Cdk2 (37). Consequently, functional redundancies by Cdk1 or Cdk4/6 may compensate for the lack of Cdk2 in those cell types. For example, Cdk1 was shown to bind cyclin E in various tissues and also to regulate G1/S phase transition in mouse embryonic fibroblasts (38). However, we show here that while Cdk2 is dispensable for mammary gland development, it is required for LMW cyclin E tumorigenesis. This reflects the requirement for the phosphorylation of a specific set of substrates that mediates the oncogenic function of LMW cyclin E/Cdk2 kinase. This result suggests that targeting LMW cyclin E/Cdk2 kinase activity may be a viable and specific strategy for clinical treatment of certain breast tumors.
Our experiments show that short-term exposure to roscovitine or meriolin results in a significant delay of LMW-cyclin E induced mammary tumor development. This delay may be due to decreased proliferation and partial induction of cell death following treatment. These results also suggest that the LMW cyclin E transgenic mouse model should be a valuable tool to specifically evaluate drugs against the oncogenic LMW cyclin E/ Cdk2 kinase frequently expressed in triple negative breast cancers. Future studies will aim at identifying the targets of the oncogenic LMW cyclin E/Cdk2 kinase that provide breast cancer cells with the capabilities to resist cell death.
We also propose that breast cancer patients whose tumor express the LMW cyclin E will be most responsive to Cdk2 inhibitors such as roscovitine or its analogues. Several CDK inhibitors have entered clinical trials including flavopiridol and seliciclib (roscovitine). Phase I studies have demonstrated that these drugs can generally be administrated safely (39, 40). Phase II studies with CDK inhibitors have shown little single-agent activity in solid tumors including metastatic melanoma (41), and endometrial adenocarcinoma (42) but combination with chemotherapy seems more efficient (43-45). Overall, the results from clinical trials of CDK inhibitors in breast cancer have been disappointing.
When introducing novel treatment strategies in the clinic, special attention has to be made toward identifying the patient population who will respond most effectively to such treatment. Additionally, particular attention has to be made to the mechanism of action of the agent in order to decipher the best treatment modality (i.e single agent or in combination). Specifically, for roscovitine, only those patients whose tumors express LMW cyclin E will be most responsive to this treatment.
Our study presented here shows that CDK2 kinase inhibitor is preferentially effective in LMW cyclin E overexpressing breast cancer and reinforce the need for a more targeted approach for the evaluation of this class of drugs to subgroups of breast cancer patients with high LMW cyclin E expression. Our in vitro data show that the two CDK inhibitors used are cytotoxic for breast cancer cells expressing LMW cyclin E so that we could expect to reach the same level of clinical efficacy as observed for hematologic malignancies that are more sensitive to induction of apoptosis (46, 47). It should be noted that none of the patients treated with Selicilib (Roscovitine) (in either phase I or II) were pre-selected based on altered expression of cyclin E or Cdk2. As a result, the clinical experience with Selicilib has been un-impressive. If patients are pre-selected based on their cyclin E expression their responses will likely improve.
Acknowledgments
The research in the authors' laboratory was supported by grants from the National Cancer Institute (CA87548 and P50CA116199 to K.K.) and from the Susan G Komen Breast Cancer Foundation (BCTR0504346 to S.A.).
Footnotes
Conflict of interest: All authors declare no conflict of interest.
References
- 1.Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 2.Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776–86. doi: 10.1038/sj.onc.1208613. [DOI] [PubMed] [Google Scholar]
- 3.Caldon CE, Sutherland RL, Musgrove EA. Cell cycle proteins in epithelial cell differentiation: Implications for breast cancer. Cell Cycle. 9 doi: 10.4161/cc.9.10.11474. [DOI] [PubMed] [Google Scholar]
- 4.Caldon CE, Musgrove EA. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 5:2. doi: 10.1186/1747-1028-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DC, Zhang N, Danes C, et al. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol. 2001;21:6254–69. doi: 10.1128/MCB.21.18.6254-6269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akli S, Zheng PJ, Multani AS, et al. Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res. 2004;64:3198–208. doi: 10.1158/0008-5472.can-03-3672. [DOI] [PubMed] [Google Scholar]
- 7.Bagheri-Yarmand R, Nanos-Webb A, Biernacka A, Bui T, Keyomarsi K. Cyclin E deregulation impairs mitotic progression through premature activation of Cdc25C. Cancer Res. 70:5085–95. doi: 10.1158/0008-5472.CAN-09-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagheri-Yarmand R, Biernacka A, Hunt KK, Keyomarsi K. Low molecular weight cyclin E overexpression shortens mitosis, leading to chromosome missegregation and centrosome amplification. Cancer Res. 70:5074–84. doi: 10.1158/0008-5472.CAN-09-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–75. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 10.Akli S, Van Pelt CS, Bui T, et al. Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res. 2007;67:7212–22. doi: 10.1158/0008-5472.CAN-07-0599. [DOI] [PubMed] [Google Scholar]
- 11.Wingate H, Bedrosian I, Akli S, Keyomarsi K. The low molecular weight (LMW) isoforms of cyclin E deregulate the cell cycle of mammary epithelial cells. Cell Cycle. 2003;2:461–6. [PubMed] [Google Scholar]
- 12.Akli S, Bui T, Wingate H, et al. Low-molecular-weight cyclin E can bypass letrozole-induced G1 arrest in human breast cancer cells and tumors. Clin Cancer Res. 16:1179–90. doi: 10.1158/1078-0432.CCR-09-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma T, Van Tine BA, Wei Y, et al. Cell cycle-regulated phosphorylation of p220(NPAT) by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 2000;14:2298–313. doi: 10.1101/gad.829500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuda M, Horn HF, Tarapore P, et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell. 2000;103:127–40. doi: 10.1016/s0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev Cell. 2002;3:339–50. doi: 10.1016/s1534-5807(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 16.Grimison B, Liu J, Lewellyn AL, Maller JL. Metaphase arrest by cyclin E-Cdk2 requires the spindle-checkpoint kinase Mps1. Curr Biol. 2006;16:1968–73. doi: 10.1016/j.cub.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 17.Ruffner H, Jiang W, Craig AG, Hunter T, Verma IM. BRCA1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol Cell Biol. 1999;19:4843–54. doi: 10.1128/mcb.19.7.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazumder S, Plesca D, Almasan A. A jekyll and hyde role of cyclin E in the genotoxic stress response: switching from cell cycle control to apoptosis regulation. Cell Cycle. 2007;6:1437–42. doi: 10.4161/cc.6.12.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng Y, Yu Q, Sicinska E, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–43. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 20.Parisi T, Beck AR, Rougier N, et al. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003;22:4794–803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng Y, Lee YM, Welcker M, et al. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–39. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Berger C, Pallavi SK, Prasad M, Shashidhara LS, Technau GM. A critical role for cyclin E in cell fate determination in the central nervous system of Drosophila melanogaster. Nat Cell Biol. 2005;7:56–62. doi: 10.1038/ncb1203. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 2004;306:885–8. doi: 10.1126/science.1103544. [DOI] [PubMed] [Google Scholar]
- 24.Ortega S, Prieto I, Odajima J, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 25.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Lowe M, Herliczek TW, Keyomarsi K. Lovastatin mediated G1 arrest in normal and tumor breast cells is through inhibition of CDK2 activity and redistribution of p21 and p27, independent of p53. Oncogene. 1998;17:2393–402. doi: 10.1038/sj.onc.1202322. [DOI] [PubMed] [Google Scholar]
- 27.Keyomarsi K, Pardee AB. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci U S A. 1993;90:1112–6. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keyomarsi K, Conte D, Jr, Toyofuku W, Fox MP. Deregulation of cyclin E in breast cancer. Oncogene. 1995;11:941–50. [PubMed] [Google Scholar]
- 29.Brooks EE, Gray NS, Joly A, et al. CVT-313, a specific and potent inhibitor of CDK2 that prevents neointimal proliferation. J Biol Chem. 1997;272:29207–11. doi: 10.1074/jbc.272.46.29207. [DOI] [PubMed] [Google Scholar]
- 30.Bettayeb K, Tirado OM, Marionneau-Lambot S, et al. Meriolins, a new class of cell death inducing kinase inhibitors with enhanced selectivity for cyclin-dependent kinases. Cancer Res. 2007;67:8325–34. doi: 10.1158/0008-5472.CAN-07-1826. [DOI] [PubMed] [Google Scholar]
- 31.Meijer L, Borgne A, Mulner O, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–36. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 32.Wingate H, Zhang N, McGarhen MJ, Bedrosian I, Harper JW, Keyomarsi K. The tumor-specific hyperactive forms of cyclin E are resistant to inhibition by p21 and p27. J Biol Chem. 2005;280:15148–57. doi: 10.1074/jbc.M409789200. [DOI] [PubMed] [Google Scholar]
- 33.McClue SJ, Blake D, Clarke R, et al. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine) Int J Cancer. 2002;102:463–8. doi: 10.1002/ijc.10738. [DOI] [PubMed] [Google Scholar]
- 34.Echalier A, Bettayeb K, Ferandin Y, et al. Meriolins (3-(pyrimidin-4-yl)-7-azaindoles): synthesis, kinase inhibitory activity, cellular effects, and structure of a CDK2/cyclin A/meriolin complex. J Med Chem. 2008;51:737–51. doi: 10.1021/jm700940h. [DOI] [PubMed] [Google Scholar]
- 35.Berthet C, Rodriguez-Galan MC, Hodge DL, et al. Hematopoiesis and thymic apoptosis are not affected by the loss of Cdk2. Mol Cell Biol. 2007;27:5079–89. doi: 10.1128/MCB.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandenbosch R, Borgs L, Beukelaers P, et al. CDK2 is dispensable for adult hippocampal neurogenesis. Cell Cycle. 2007;6:3065–9. doi: 10.4161/cc.6.24.5048. [DOI] [PubMed] [Google Scholar]
- 37.Jablonska B, Aguirre A, Vandenbosch R, et al. Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone. J Cell Biol. 2007;179:1231–45. doi: 10.1083/jcb.200702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleem E, Kiyokawa H, Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol. 2005;7:831–6. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- 39.Fekrazad HM, Verschraegen CF, Royce M, Smith HO, Chyi Lee F, Rabinowitz I. A phase I study of flavopiridol in combination with gemcitabine and irinotecan in patients with metastatic cancer. Am J Clin Oncol. 2010;33:393–7. doi: 10.1097/COC.0b013e3181b2043f. [DOI] [PubMed] [Google Scholar]
- 40.Benson C, White J, De Bono J, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer. 2007;96:29–37. doi: 10.1038/sj.bjc.6603509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdette-Radoux S, Tozer RG, Lohmann RC, et al. Phase II trial of flavopiridol, a cyclin dependent kinase inhibitor, in untreated metastatic malignant melanoma. Invest New Drugs. 2004;22:315–22. doi: 10.1023/B:DRUG.0000026258.02846.1c. [DOI] [PubMed] [Google Scholar]
- 42.Grendys EC, Jr, Blessing JA, Burger R, Hoffman J. A phase II evaluation of flavopiridol as second-line chemotherapy of endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;98:249–53. doi: 10.1016/j.ygyno.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 43.El-Rayes BF, Gadgeel S, Parchment R, Lorusso P, Philip PA. A phase I study of flavopiridol and docetaxel. Invest New Drugs. 2006;24:305–10. doi: 10.1007/s10637-005-4343-5. [DOI] [PubMed] [Google Scholar]
- 44.Fornier MN, Rathkopf D, Shah M, et al. Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clin Cancer Res. 2007;13:5841–6. doi: 10.1158/1078-0432.CCR-07-1218. [DOI] [PubMed] [Google Scholar]
- 45.Bible KC, Lensing JL, Nelson SA, et al. Phase 1 trial of flavopiridol combined with cisplatin or carboplatin in patients with advanced malignancies with the assessment of pharmacokinetic and pharmacodynamic end points. Clin Cancer Res. 2005;11:5935–41. doi: 10.1158/1078-0432.CCR-04-2566. [DOI] [PubMed] [Google Scholar]
- 46.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–45. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]