Fig. 1.
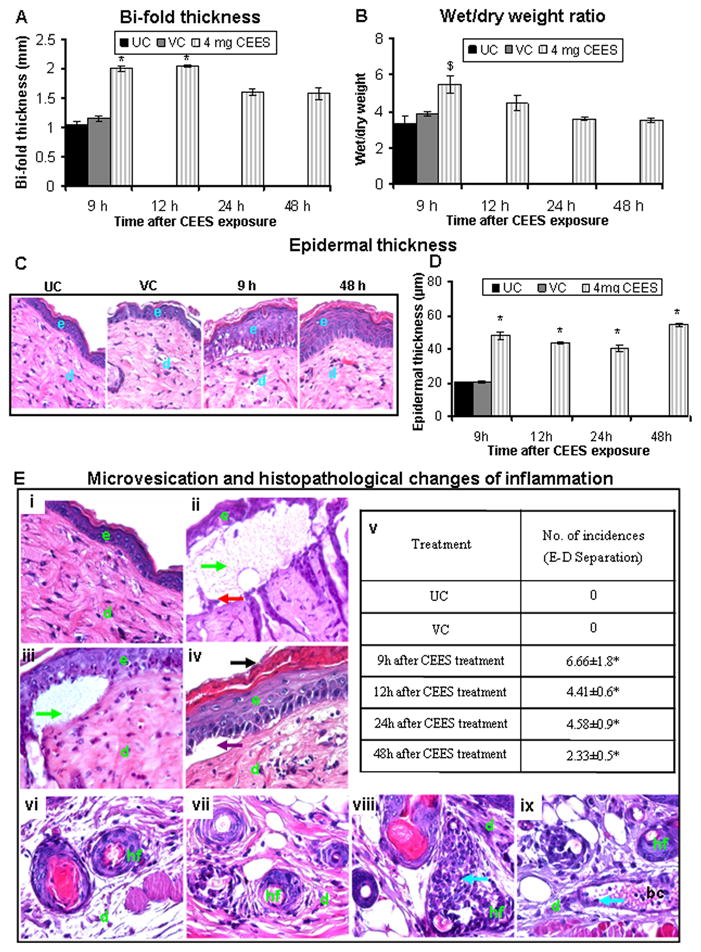
CEES topical exposure at a 4 mg dose causes inflammation-related histopathological alterations and microvesication in male SKH-1 hairless mouse skin. Following 4 mg CEES and control exposures, skin bi-fold thickness (A) and wet/dry weight ratio (B) were recorded as detailed under Materials and Methods. Representative H&E stained skin tissue sections from untreated control, vehicle control, and 4 mg CEES exposed mice at 9 to 48 h post exposure showing changes in epidermal thickness (×400 magnification; C) and quantitated epidermal thickness (D). Representative H&E stained skin sections from untreated (i and vi) and vehicle (vii) controls, 4 mg CEES treatment for 9 h (ii and viii), 12 h (iii and ix), and number of epidermal-dermal separations incidences (v) per 6 cm2 field, ×400 magnification (E); (iv) shows the artifact of microvesication because it is not filled with protein exudate and not counted as true microvesication. Data presented are mean ± SEM of four-five animals in each treatment group. Statistical significance of difference between CEES and control groups were determined by one way ANOVA followed by Bonferroni t-test for pair wise multiple comparisons. *, p<0.001; $, p<0.01 and #, p<0.05 as compared to untreated control. UC, untreated control; VC, vehicle control; e, epidermis; d, dermis; hf, hair follicle; bc, blood capillary; green arrows, epidermal-dermal separations (microvesication); black arrow, parakeratosis; violet arrow, artifact (not microvesication); red arrow, hemidesmosomal components; and blue arrows, neutrophils.
