Abstract
Glucocorticoids are the most effective anti-inflammatory therapy for asthma yet are relatively ineffective in chronic obstructive pulmonary disease. Glucocorticoids suppress inflammation via several molecular mechanisms. Glucocorticoids suppress the multiple inflammatory genes that are activated in chronic inflammatory diseases, such as asthma, by reversing histone acetylation of activated inflammatory genes through binding of ligand-bound glucocorticoid receptors (GR) to co-activator molecules and recruitment of histone deacetylase-2 to the activated inflammatory gene transcription complex (trans-repression). At higher concentrations of glucocorticoids GR homodimers interact with DNA recognition sites to activate transcription through increased histone acetylation of anti-inflammatory genes and transcription of several genes linked to glucocorticoid side effects (trans-activation). Glucocorticoids also have post-transcriptional effects and decrease stability of some pro-inflammatory mRNA species. Decreased glucocorticoid responsiveness is found in patients with severe asthma and asthmatics who smoke, as well as in all patients with chronic obstructive pulmonary disease. Several molecular mechanisms of glucocorticoid resistance have now been identified which involve post-translational modifications of GR. Histone deacetylase-2 is markedly reduced in activity and expression as a result of oxidative/nitrative stress so that inflammation becomes resistant to the anti-inflammatory actions of glucocorticoids. Dissociated glucocorticoids and selective GR modulators which show improved trans-repression over trans-activation effects have been developed to reduce side effects, but so far it has been difficult to dissociate anti-inflammatory effects from adverse effects. In patients with glucocorticoid resistance alternative anti-inflammatory treatments are being investigated as well as drugs that may reverse the molecular mechanisms of glucocorticoid resistance.
LINKED ARTICLES
This article is part of a themed issue on Respiratory Pharmacology. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-1
Keywords: asthma, COPD, inflammation, p38 MAP kinase, histone deacetylase, steroid resistance, oxidative stress, β2-adrenoceptors
Introduction
Glucocorticosteroids (also called glucocorticoids, corticosteroids or steroids) are the most effective anti-inflammatory drugs available for the treatment of many chronic inflammatory and immune diseases, including asthma. However, a minority of patients with these diseases show little or no response even to high doses of glucocorticoids. Several other inflammatory diseases, including chronic obstructive pulmonary disease (COPD), interstitial pulmonary fibrosis and cystic fibrosis, appear to be largely glucocorticoid-resistant. Both asthma and COPD involve chronic inflammation of the respiratory tract, with the activation and recruitment of many inflammatory cells and orchestrated by a complex network of inflammatory mediators (Barnes, 2008a,b;). However, there are differences in the nature of this inflammation and its inflammatory consequences between these diseases and perhaps this is best demonstrated by the differing response to glucocorticoids, which is excellent in most patients with asthma but very poor in most patients with COPD. There is now a much better understanding of how glucocorticoids suppress chronic inflammation in asthma and also why they fail to work in some patients with asthma and most patients with COPD, despite the fact that inflammatory genes are activated in these two diseases by similar molecular mechanisms. This has given insights into how glucocorticoids might be improved in the future and how glucocorticoid resistance may be overcome with new classes of therapy (Barnes and Adcock, 2009).
Clinical use in asthma
The early use of inhaled corticosteroids (ICS) has revolutionized the management of asthma, with marked reductions in asthma morbidity and mortality. ICS are now recommended as first-line therapy for all patients with persistent asthma, including children (Bateman et al., 2008). Several topically acting glucocorticoids are now available for inhalation and all have similar efficacy, but have pharmacokinetic differences that account for differences in therapeutic ratio between these drugs. ICS are very effective in controlling asthma symptoms in asthmatic patients of all ages and severity. ICS improve the quality of life of patients with asthma and allow many patients to lead normal lives, improve lung function, reduce the frequency of exacerbations and may prevent irreversible airway changes (Barnes et al., 1998; O'Byrne et al., 2006). ICS were first introduced to reduce the requirement for oral glucocorticoids in patients with severe asthma and many studies have confirmed that the great majority of patients can be weaned off oral glucocorticoid. Only about 1% of asthmatic patients now require maintenance treatment with oral glucocorticoids for control of asthma (‘steroid-dependent’ asthmatics), but short courses of oral glucocorticoids are still needed to treat exacerbations of asthma. There are local side effects of ICS, including increased oral candidiasis and dysphonia, but these are rarely a major problem. Systemic side effects, largely arising from absorption of ICS from the lung, are not a problem in patients treated with the usually required doses, but may become a problem in patients with severe asthma who require larger doses for asthma control.
Clinical use in COPD
Most patients with COPD have a poor response to glucocorticoids in comparison to asthma with little improvement in lung function or symptoms (Suissa and Barnes, 2009; Barnes, 2010a). High doses of ICS have shown a reduction (20–25%) in exacerbations in patients with severe disease and this is the main clinical indication for their use (Rabe et al., 2007). However, even the effect on exacerbations has been questioned as it is largely explained by an artefact in trial design (Suissa and Barnes, 2009). Several large studies have shown that glucocorticoids failed to reduce the progression in COPD [measured by annual fall in forced expiratory volume in 1 second (FEV1) ] (Yang et al., 2007) and failed to reduce mortality in a large study where this was the primary outcome measure (Calverley et al., 2007). These results are likely to reflect the resistance of pulmonary inflammation to glucocorticoid in COPD patients which is discussed below. Current guidelines suggest that high doses of ICS should be used only in patients with severe disease (FEV1 < 50% predicted) who have frequent exacerbations (≤2 per year) which would comprise about 10% of patients, whereas currently high-dose ICS are used in approximately 80% of patients with a clinical diagnosis of COPD. This overuse of glucocorticoids is likely to produce several long-term side effects, such as osteoporosis, diabetes, cataracts, hypertension and pneumonia (Barnes, 2010a). Oral glucocorticoids are used to treat acute exacerbations, although they are poorly effective. Some patients with COPD, who also have concomitant asthma (termed ‘overlap syndrome’), benefit from ICS and these patients may be recognized by increased sputum eosinophils and exhaled nitric oxide and by a greater bronchodilator reversibility (Gibson and Simpson, 2009).
Anti-inflammatory mechanisms of glucocorticoids
There have been major advances in understanding the molecular mechanisms whereby glucocorticoids suppress inflammation in asthma (Rhen and Cidlowski, 2005; Barnes, 2006b; 2010b;). Glucocorticoids activate many anti-inflammatory genes, and repress many pro-inflammatory genes that have been activated in inflammation (Table 1), as well as having several post-transcriptional effects. Understanding the molecular mechanisms of glucocorticoid action has also provided new insights into understanding molecular mechanisms involved in glucocorticoid resistance (Barnes and Adcock, 2009).
Table 1.
Effect of glucocorticoids on transcription of genes relevant to asthma
| Increased transcription (trans-activation) |
| •Lipocortin-1 |
| •β2-Adrenoceptors |
| •Secretory leukocyte inhibitory protein |
| •IκB-α (inhibitor of NF-κB) |
| •MKP1 (inhibits MAP kinase pathways) |
| •Glucocorticoid inducible leucine zipper (GILZ) |
| •Anti-inflammatory or inhibitory cytokines |
| IL-10, IL-12, IL-1 receptor antagonist |
| Decreased transcription (trans-repression) |
| •Inflammatory cytokines |
| IL-2, IL-3, IL-4, IL-5, IL-6, IL-13, IL-15, TNF-α, GM-CSF, SCF, TSLP |
| •Chemokines |
| CCL1, CCL5, CCL11, CXCL8 |
| •Inflammatory enzymes |
| Inducible nitric oxide synthase (iNOS), inducible cyclo-oxygenase (COX-2) |
| Inducible phospholipase A2 (cPLA2) |
| •Inflammatory peptides |
| Endothelin-1 |
| •Mediator Receptors |
| Neurokinin (NK1)-, bradykinin (B2)-receptors |
| •Adhesion molecules |
| ICAM-1,VCAM-1 |
ICAM-1, intercellular cell adhesion molecule 1; IL, interleukin; MAP, mitogen-activated protein; NF-κB, nuclear factor-Kb; TNF-α, tumour necrosis factor α; VCAM-1, vascular cell adhesion molecule 1.
Glucocorticoid receptors (GR)
Glucocorticoids diffuse across the cell membrane and bind to GR in the cytoplasm (Rhen and Cidlowski, 2005; Nicolaides et al., 2010). Upon ligand binding, GR are activated and released from chaperone proteins (heat shock protein-90 and others) and rapidly translocate to the nucleus where they exerts their molecular effects. The mechanism of nuclear translocation involves the nuclear import proteins importin-α (karyopherin-β) and importin-13. (Goldfarb et al., 2004; Tao et al., 2006). There is only one form of GR that binds glucocorticoids termed GRα. GRβ is an alternatively spliced form of GR that interacts with DNA but not with glucocorticoids, so may theoretically act as a dominant-negative inhibitor of glucocorticoid action by interfering with the binding of GR to DNA (Lewis-Tuffin and Cidlowski, 2006). In addition, there is evidence that multiple GR isoforms are generated by alternative splicing and alternative translation initiation. These isoforms have unique tissue distribution patterns and transcriptional regulatory profiles. Furthermore, each is subject to various post-translational modifications that may affect receptor function, which determine the cell-specific response to glucocorticoids (Lu and Cidlowski, 2004).
Gene activation
Glucocorticoid receptors homodimerize and bind to glucocorticoid response elements (GRE) in the promoter region of glucocorticoid-responsive genes and this interaction switches on (or occasionally switches off) gene transcription. Activation of glucocorticoid-responsive genes occurs via an interaction between the DNA-bound GR and transcriptional co-activator molecules such as CREB-binding protein, which have intrinsic histone acetyltransferase activity and cause acetylation of core histones (particularly histone-4). This tags histones to recruit chromatin remodelling engines such as SWI/SNF and subsequent association of RNA polymerase II resulting in gene activation (Figure 1) (Ito et al., 2000; John et al., 2008). Genes that are switched on by glucocorticoids include genes encoding β2-adrenergic receptors and the anti-inflammatory proteins secretory leukoprotease inhibitor and mitogen-activated protein kinase phosphatase-1 (MKP-1), which inhibits mitogen-activated protein (MAP) kinase pathways. These effects may contribute to the anti-inflammatory actions of glucocorticoids (Clark, 2003; Barnes, 2006a). GR interaction with negative GREs, or to GREs that cross the transcriptional start site, may suppress gene transcription and this may be important in mediating many of the side effects of glucocorticoids, such as inhibition of osteocalcin that is involved in bone synthesis (Figure 2) (Dostert and Heinzel, 2004).
Figure 1.
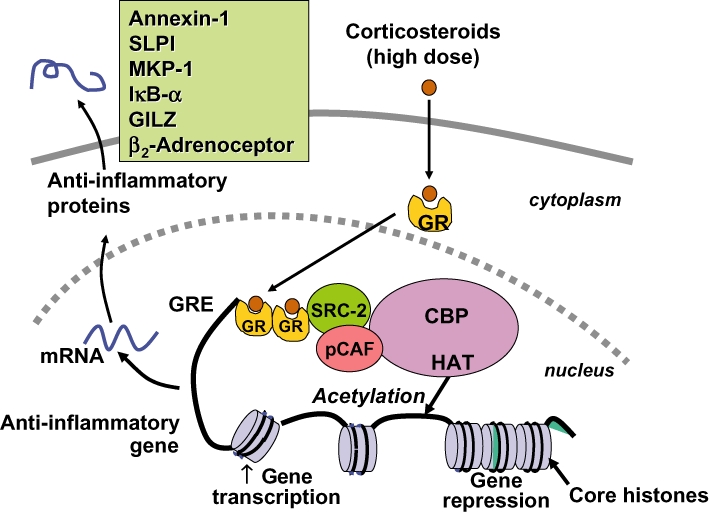
Glucocorticoid activation of anti-inflammatory gene expression. Glucocorticoids bind to cytoplasmic glucocorticoid receptors (GR) which translocate to the nucleus where they bind to glucocorticoid response elements (GRE) in the promoter region of steroid-sensitive genes and also directly or indirectly to co-activator molecules such as CREB-binding protein (CBP), p300/CBP activating factor (pCAF) or steroid receptor coactivator-2 (SRC-2), which have intrinsic histone acetyltransferase (HAT) activity, causing acetylation of lysines on histone H4, which leads to activation of genes encoding anti-inflammatory proteins, such as secretory leukoprotease inhibitor (SLPI), mitogen-activated kinase phosphatase-1 (MKP-1), inhibitor of nuclear factor κB (IκB-α) and glucocorticoid-induced leucine zipper protein (GILZ).
Figure 2.
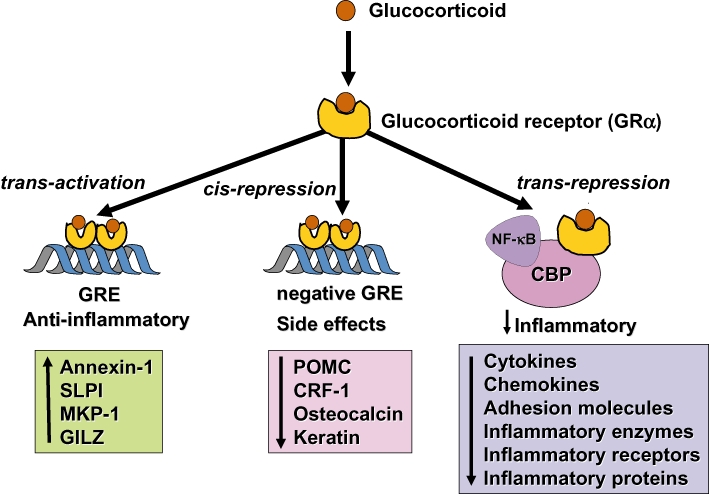
Glucocorticoids regulate gene expression in several ways. Glucocorticoids enter the cell to bind to glucocorticoid receptors (GR) in the cytoplasm that translocate to the nucleus. GR homodimers bind to glucocorticoid-response elements (GRE) in the promoter region of steroid-sensitive genes, which may encode anti-inflammatory proteins. Less commonly, GR homodimers interact with negative GREs to suppress genes. Nuclear GR also interact with co-activator molecules, such as CREB-binding protein (CBP), which is activated by pro-inflammatory transcription factors, such as nuclear factor-κB (NF-κB), thus switching off the inflammatory genes that are activated by these transcription factors. Other abbreviations: CRF, corticotrophin releasing factor; GILZ, glucocorticoid-induced leucine zipper protein; IκB-α, inhibitor of NF-κB; MKP-1, mitogen-activated kinase phosphatase-1; POMC, proopiomelanocortin; SLPI, secretory leukoprotease inhibitor.
Switching off activated inflammatory genes
The major action of glucocorticoids is to switch off multiple activated inflammatory genes that encode for cytokines, chemokines, adhesion molecules inflammatory enzymes and receptors (Barnes and Adcock, 2003). These genes are switched on in the airways by pro-inflammatory transcription factors, such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), both of which are usually activated at sites of inflammation in asthma and COPD, resulting in the switching on of multiple inflammatory genes. These genes are activated through interactions with transcriptional co-activator molecules in a similar manner to that described above for GR-mediated gene transcription (Barnes et al., 2005).
Activated GR interact with corepressor molecules to attenuate NF-κB-associated co-activator activity, thus reducing histone acetylation, chromatin remodelling and RNA polymerase II actions (Ito et al., 2000; Barnes, 2006b). Reduction of histone acetylation more importantly occurs through the specific recruitment of histone deacetylase-2 (HDAC2) to the activated inflammatory gene complex by activated GR, thereby resulting in effective suppression of activated inflammatory genes within the nucleus (Figure 3). This may account for not only why glucocorticoids are so effective in the control of inflammation, but also why they are relatively safe, because genes other than those that encode inflammatory proteins are not affected. GR becomes acetylated upon ligand binding allowing it to bind to GREs and HDAC2 can target acetylated GR thereby allowing it to associate with the NF-κB complex (Ito et al., 2006) (Figure 4). The site of acetylation of GR is the lysine rich region -492-495 with the sequence KKTK, which is analogous to the acetylation sites identified on other nuclear hormone receptors. Site-directed mutagenesis of the lysine residues K494 and K495 prevents GR acetylation and reduces the activation of the SLPI gene by glucocorticoids, whereas repression of NF-κB is unaffected. HDAC6 has also been implicated in GR function by modulating hsp90 acetylation status and thereby GR nuclear translocation (Kovacs et al., 2005).
Figure 3.
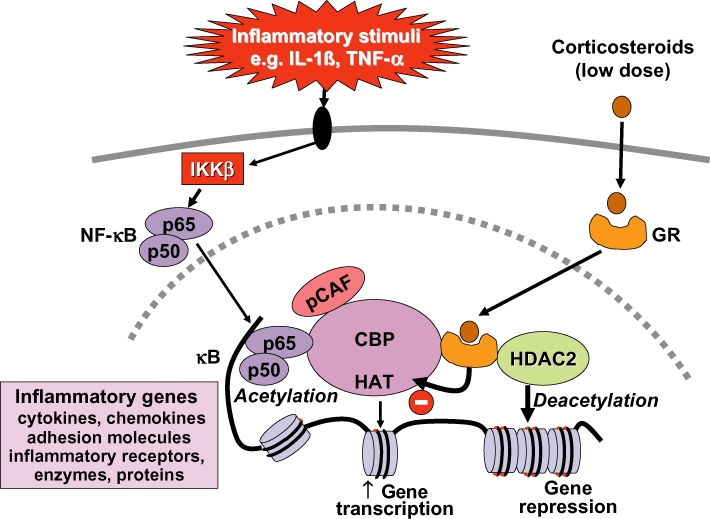
Glucocorticoid suppression of activated inflammatory genes. Inflammatory genes are activated by inflammatory stimuli, such as interleukin-1β (IL-1β) or tumour necrosis factor-α (TNF-α), resulting in activation of IKKβ (inhibitor of I-κB kinase-β), which activates the transcription factor nuclear factor κB (NF-κB). A dimer of p50 and p65 NF-κB proteins translocates to the nucleus and binds to specific κB recognition sites and also to co-activators, such as CREB-binding protein (CBP) or p300/CBP-activating factor (pCAF), which have intrinsic histone acetyltransferase (HAT) activity. This results in acetylation of core histone H4, resulting in increased expression of genes encoding multiple inflammatory proteins. Glucocorticoid receptors (GR) after activation by glucocorticoids translocate to the nucleus and bind to co-activators to inhibit HAT activity directly and recruiting histone deacetylase-2 (HDAC2), which reverses histone acetylation leading in suppression of these activated inflammatory genes.
Figure 4.
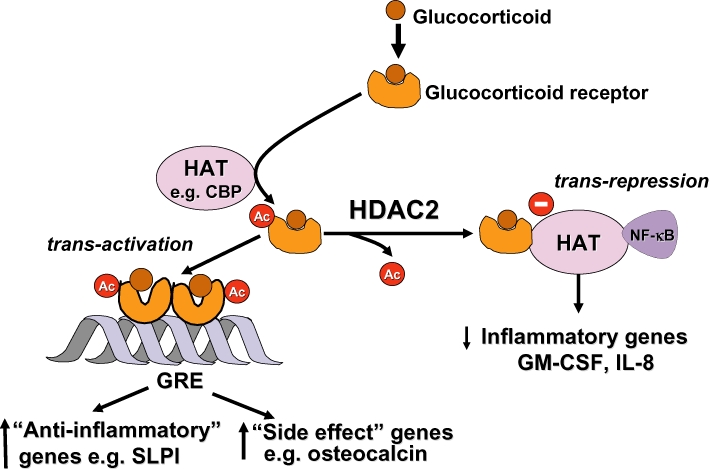
Acetylation of glucocorticoid receptors (GR). Binding of a glucocorticoids to GR results in its acetylation by histone acetyltransferases (HAT), such as CREB-binding protein (CBP), and a dimer of acetylated GR then binds to glucocorticoid response elements (GRE) to activate or suppress genes (such as side effect genes). Deacetylation of GR by histone deacetylase-2 (HDAC2) is necessary for GR to interact with CBP and inhibit nuclear factor-κB (NF-κB) to switch off inflammatory genes. GM-CSF, granulocyte-macrophage colony stimulating factor; IL, interleukin.
Additional mechanisms are also important in the anti-inflammatory actions of glucocorticoids. Glucocorticoids have potent inhibitory effects on mitogen-activated protein kinase (MAPK) signalling pathways through the induction of MKP-1 and this may inhibit the expression of multiple inflammatory genes (Clark, 2003; Barnes, 2006a) (Figure 5). An important effect of glucocorticoids in the treatment of allergic diseases is through suppression of Th2 cells and Th2 cytokines [interleukin (IL)4, IL-5 and IL-13] and this may be mediated via inhibition of the transcription factor GATA3 which regulates the transcription of Th2 cytokine genes (Maneechotesuwan et al., 2007). This is controlled by translocation of GATA3 from the cytoplasm to the nucleus via importin-α after phosphorylation by p38 MAPK. Glucocorticoids potently inhibit GATA3 nuclear translocation as GR competes for nuclear import via importin-α and also induces MKP-1 to reverse the phosphorylation of GATA3 by p38 MAPK (Maneechotesuwan et al., 2009). A further immunosuppressive effect of glucocorticoids is through enhanced activity and expression of indoleamine-2,3-dioxygenase, a tryptophan-degrading enzyme that plays a key role in the regulation of T-lymphocyte function in allergic diseases through increased secretion of the anti-inflammatory cytokine IL-10 (Maneechotesuwan et al., 2008). Interestingly, this effect of glucocorticoids on indoleamine-2,3-dioxygenase is further enhanced by statins (Maneechotesuwan et al., 2010).
Figure 5.
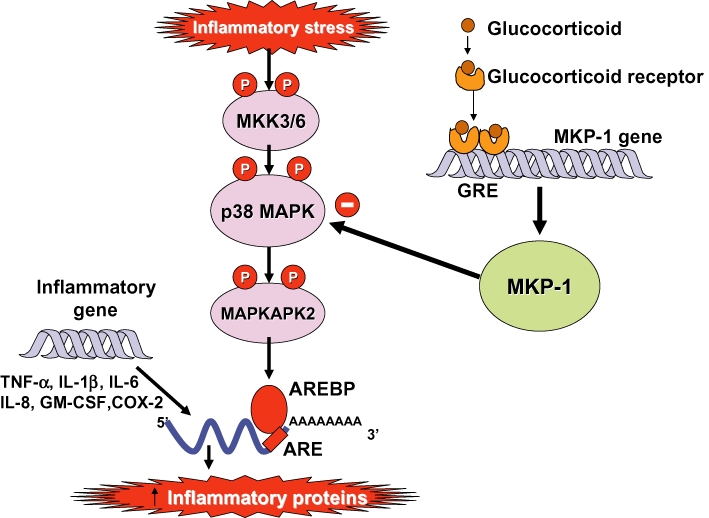
Inhibition of p38 mitogen-activated protein (MAP) kinase by glucocorticoids. p38 MAP kinase is activated by inflammatory stresses though activation of MAP kinase kinase (MKK)-3 and -6. p38 phosphorylates (P) MAP kinase-activated protein kinase(MAPKAPK)-2, which plays a role in stabilizing messenger RNA (mRNA) encoding several inflammatory proteins, such as tumour necrosis factor-α (TNF-α), interleukin(IL)-1β, IL-6, IL-8, granulocyte-macrophage colony stimulating factor (GM-CSF) and cyclo-oxygenase(COX)-2. This mRNA is characterized by AU-rich elements (ARE) in the 3'-untranslated region, which make the mRNA unstable and rapidly degraded. ARE-binding proteins (AREBP) stabilize these proteins and may be activated (probably indirectly) by MAPKAPK-2. Corticosteroids induce the expression of MAP kinase phosphatase (MKP)-1, which inhibits p38 and thus prevents the stabilization of multiple inflammatory proteins.
Post-transcriptional effects
Some pro-inflammatory genes, such as tumour necrosis factor-α (TNF-α), have unstable messenger RNA that is rapidly degraded by certain RNAses but stabilized when cells are stimulated by inflammatory mediators. Glucocorticoids reverse this effect, resulting in rapid degradation of mRNA and reduced inflammatory protein secretion (Bergmann et al., 2004) (Figure 5). This may be mediated through the increased gene expression of proteins that destabilize mRNAs of inflammatory proteins, such as the zinc finger protein tristetraprolin, which binds to the 3' AU-rich untranslated region of mRNAs (Smoak and Cidlowski, 2006).
Cellular effects in asthma and COPD
At a cellular level glucocorticoids reduce the numbers of inflammatory cells in the airways, including eosinophils, T-lymphocytes, mast cells and dendritic cells (Barnes et al., 1998). These effects of glucocorticoids are produced through inhibiting the recruitment of inflammatory cells into the airway by suppressing the production of chemotactic mediators and adhesion molecules and by inhibiting the survival in the airways of inflammatory cells, such as eosinophils, T-lymphocytes and mast cells. Epithelial cells may be the major cellular target for ICS, which are the mainstay of modern asthma management. ICS suppress many activated inflammatory genes in airway epithelial cells. Epithelial integrity is restored by regular ICS (Barnes et al., 1998). The suppression of mucosal inflammation is relatively rapid with a significant reduction in eosinophils detectable within 3 h and associated with reduced airway hyperresponsiveness (Gibson et al., 2001; Ketchell et al., 2002; Erin et al., 2008). This almost certainly accounts for the clinical benefits seen with inhalation of budesonide and formoterol combination inhaler as a rescue therapy in asthma, as this is likely to stop the evolution of an exacerbation (Barnes, 2007). However, reversal of airway hyperresponsiveness may take several months to reach a plateau, probably reflecting recovery of structural changes in the airway (Juniper et al., 1990).
In COPD patients even high doses of ICS fail to reduce airway inflammation. This glucocorticoid resistance has been demonstrated by the failure of high doses of ICS to reduce inflammatory markers in sputum or bronchial biopsies of COPD patients (Keatings et al., 1997; Culpitt et al., 1999; Loppow et al., 2001; Hattotuwa et al., 2002; Bourbeau et al., 2007). The reason why ICS fail to suppress inflammation cannot be explained by impaired access of the inhaled drug to sites of inflammation as an oral glucocorticoid is equally ineffective (Keatings et al., 1997).
Interaction with β2-adrenergic receptors
Inhaled β2-agonists and glucocorticoids are frequently used together (usually as a fixed combination inhaler containing a glucocorticoid with a long-acting β2-agonist) in the control of asthma and it is now recognized that there are important molecular interactions between these two classes of drug (Barnes, 2002; Giembycz et al., 2008; Newton et al., 2010). Glucocorticoids increase the transcription of the β2-receptor gene, resulting in increased expression of cell surface receptors. This has been demonstrated in human lung in vitro (Mak et al., 1995a) and nasal mucosa in vivo after topical application of a glucocorticoid (Baraniuk et al., 1997). In this way glucocorticoids protect against the down-regulation of β2-receptors after long-term administration (Mak et al., 1995b). This may be important for the non-bronchodilator effects of β2-agonists, such as mast cell stabilization. Glucocorticoids may also enhance the coupling of β2-receptors to G-protein (Gs), thus enhancing β2-agonist effects and reversing the uncoupling of β2-receptors that may occur in response to inflammatory mediators, such as IL-1β through a stimulatory effect on a G-protein coupled receptor kinase (Mak et al., 2002).
There is now increasing evidence that β2-agonists may affect GR function and thus enhance the anti-inflammatory effects of glucocorticoids. Long-acting β2-Agonists increase the translocation of GR from cytoplasm to the nucleus after activation by glucocorticoids (Roth et al., 2002). This effect has now been demonstrated in sputum macrophages of asthmatic patients after an ICS and inhaled long-acting β2-agonist (Usmani et al., 2005). This suggests that long-acting β2-agonists and glucocorticoids enhance each others' beneficial effects in asthma therapy and this may contribute to the greater efficacy of combination inhalers compared with increased doses of ICS in clinical trials (Gibson et al., 2007).
Glucocorticoid resistance
Patients with severe asthma have a poor response to glucocorticoids, which necessitates the need for high doses and a very small number of patients are completely resistant. These patients are difficult to manage as they get side effects from high doses of glucocorticoids, despite their lack of clinical benefit. All patients with COPD show a degree of glucocorticoid resistance (Barnes, 2010a). Asthmatics who smoke are also relatively glucocorticoid-resistant and require increased doses of glucocorticoids for asthma control (Thomson and Spears, 2005; Ahmad et al., 2008). Several molecular mechanisms have now been identified to account for glucocorticoid resistance in severe asthma and COPD (Adcock and Barnes, 2008; Barnes and Adcock, 2009).
Molecular mechanisms of glucocorticoid resistance
Several distinct molecular mechanisms contributing to decreased anti-inflammatory effects of glucocorticoids have now been identified, so that there is heterogeneity of mechanisms even within a single disease (Table 2) (Adcock and Barnes, 2008; Barnes and Adcock, 2009). Similar molecular mechanisms have also been identified in different inflammatory diseases indicating that there may be common therapeutic approaches to glucocorticoid-resistant diseases in the future.
Table 2.
Molecular mechanisms of glucocorticoid resistance
| •Familial glucocorticoid resistance |
| •Glucocorticoid receptor modification |
| Phosphorylation: decreased nuclear translocation |
| p38 MAP kinase due to IL-2 + IL-4 or IL-13 in severe asthma |
| p38 MAP kinase due to MIF in several inflammatory diseases |
| JNK due to pro-inflammatory cytokines |
| ERK due to microbial superantigens |
| Nitrosylation: ↑ NO from inducible NO synthase |
| Ubiquitination: ↑ degradation by proteasome |
| •Increased GRβ expression |
| •Increased pro-inflammatory transcription factors |
| Activator protein-1, JNK |
| STAT5, JAK3 |
| •Defective histone acetylation |
| Decreased acetylation of lysine-5 on histone 4 |
| Decreased histone deacetylase-2 |
| ↑ oxidative stress |
| ↑ phosphoinositide-3-kinase-δ activation |
| •Increased P-glycoprotein |
| Increased efflux of steroids |
ERK, extracellular signal-regulated kinase; IL, interleukin; JNK, c-Jun N-terminal kinase; MAP, mitogen-activated protein; MIF, macrophage migration inhibitory factor; NO, nitric oxide; STAT, signal transduction activated transcription factor.
Genetic susceptibility
The early descriptions of glucocorticoid-resistant asthma suggested that it was more commonly found within families (Carmichael et al., 1981), indicating that genetic factors may determine glucocorticoid responsiveness. Microarray studies of peripheral blood mononuclear cells (PBMC) from glucocorticoid-sensitive and glucocorticoid-insensitive asthma patients identified 11 genes that discriminated between these patients (Hakonarson et al., 2005), suggesting that it might be possible to develop a genomic test for glucocorticoid resistance. However, in normal subjects differential gene expression between the 10% with the greatest and least glucocorticoid responsiveness of circulating genes identified 24 genes of which the most discriminant was bone morphogenetic protein receptor type II, which enhanced glucocorticoid responsiveness when transfected into cells (Donn et al., 2007).
The very rare inherited syndrome familial glucocorticoid resistance is characterized by high circulating levels of cortisol without signs or symptoms of Cushing's syndrome (Lamberts, 2001). Clinical manifestations, which may be absent, are due to an excess of non-glucocorticoid adrenal steroids, stimulated by high adrenocorticotrophin levels, resulting in hypertension with hypokalaemia and/or signs of androgen excess (usually hirsutism and menstrual abnormalities in females). Inheritance appears to be dominant with variable expression, but only about a dozen cases have so far been reported. Sporadic cases have also been described. Several abnormalities in GR function have been described in peripheral blood leukocytes or fibroblasts from patients with familial glucocorticoid resistance, including decreased binding for cortisol, reduced numbers, thermolability and an abnormality binding to DNA, all of which are due to mutations of GR. These patients are clearly different from patients with glucocorticoid-resistant inflammatory diseases and in patients with glucocorticoid-resistant asthma mutational analysis demonstrated no obvious abnormality in GR structure (Lane et al., 1994). Various single nuclear polymorphisms of GR have been linked to altered cellular responses to glucocorticoids and a polymorphism of GRβ (GR-9β) is associated with a reduced trans-repressional response to glucocorticoids (van den Akker et al., 2006). These polymorphisms have yet to be associated with glucocorticoid resistance in airway diseases, however.
Defective GR binding and translocation
There is increased expression of IL-2 and IL-4 in the airways of patients with glucocorticoid-resistant asthma (Leung et al., 1995) and in vitro these cytokines in combination reduce GR nuclear translocation and binding affinity within the nucleus of T cells (Sher et al., 1994; Irusen et al., 2002; Matthews et al., 2004). IL-13 alone mimics this effect in monocytes (Spahn et al., 1996; Irusen et al., 2002). The mechanism whereby these cytokines reduce GR function may be mediated via phosphorylation of GR by p38 MAPK and their effect is blocked by a p38 MAPK-α inhibitor (Irusen et al., 2002). In support of this p38 MAPK shows a greater degree of activation in alveolar macrophages from asthmatics with a poor response to glucocorticoids than patients who show a normal response (Bhavsar et al., 2008). GR may be phosphorylated by several kinases that may alter its binding, stability, translocation to the nucleus, binding to DNA and interaction with other proteins, such as transcription factors and molecular chaperones (Weigel and Moore, 2007). The serine residue phosphorylated by p38 MAPK is not yet certain and may be Ser226 or Ser211, or this may be an indirect effect (Irusen et al., 2002; Szatmary et al., 2004; Miller et al., 2005). IL-2 may also cause reduced nuclear translocation in murine T cells through a mechanism involving interaction to the transcription factor STAT5 under the control of JAK3 (Goleva et al., 2002). In patients with glucocorticoid-resistant asthma a large proportion show reduced nuclear translocation of GR and reduced GRE binding in PBMC following glucocorticoid exposure and this may be explained by GR phosphorylation (Matthews et al., 2004; Szatmary et al., 2004). Another MAPK c-Jun N-terminal kinase (JNK), which is activated by TNF-α and other pro-inflammatory cytokines, also directly phosphorylates GR at Ser226 and inhibits GRE binding (Ismaili and Garabedian, 2004). Microbial superantigens induce glucocorticoid resistance in T cells in vitro via activation of extracellular receptor kinase pathways, resulting in GR phosphorylation (Li et al., 2004). MKP-1 is an endogenous inhibitor of MAPK which is activated by glucocorticoids, as discussed above. Macrophages from MKP-1 gene knock-down mice show reduced anti-inflammatory responses to glucocorticoids in vitro (Abraham et al., 2006). In asthmatic patients with glucocorticoid insensitivity there is a significant reduction in MKP-1 expression in alveolar macrophages after glucocorticoid exposure and this is correlated with increased p38 MAPK activity (Bhavsar et al., 2008).
In vitro GR may be nitrosylated by nitric oxide (NO) donors resulting in reduced binding affinity for glucocorticoids (Galigniana et al., 1999). In severe asthma and COPD there is increased expression of inducible NO synthase which produces large amounts of NO that could reduce glucocorticoid responsiveness. Whether this mechanism is relevant in glucocorticoid-resistant patients has not yet been evaluated by the use if inducible NO synthase inhibitors, however. GR may also be ubiquitinated and tagged for proteasomal degradation by E3 ubiquitin ligases implying that proteasome inhibitors may increase glucocorticoid responsiveness, although this has not yet been demonstrated in glucocorticoid-resistant disease (Wallace and Cidlowski, 2001).
Increased GRβ
Increased expression of GRβ has been reported in glucocorticoid-resistant patients of several diseases, including asthma, rheumatoid arthritis and inflammatory bowel disease (Hamid et al., 1999; Sousa et al., 2000; Orii et al., 2002; Kozaci et al., 2007), but this has not been confirmed in several other studies (Gagliardo et al., 2000; Pujols et al., 2007). GRβ is induced by pro-inflammatory cytokines and has the capacity to compete for the binding of GRα to GRE, thus acting as a dominant-negative inhibitor (Webster et al., 2001). GRβ expression is also increased by microbial superantigens, such as staphylococcal enterotoxins, which may account for glucocorticoid resistance in atopic dermatitis (Fakhri et al., 2004). However, in most cell types, apart from neutrophils, the expression of GRβ is much lower than that of GRα, making this mechanism unlikely (Pujols et al., 2007). Another mechanism may be through interference with GRα nuclear translocation, because knock-down of GRβ in alveolar macrophages from glucocorticoid-resistant asthma patients results in increased GRα nuclear localization and increased glucocorticoid responsiveness (Goleva et al., 2006) While glucocorticoids do not bind to GRβ it is transcriptionally active and the GR antagonist mifepristone (RU-486) binds to GRβ, making it translocate to the nucleus, but the endogenous ligand of GRβ is currently unidentified (Lewis-Tuffin et al., 2007).
Transcription factor activation
Excessive activation of AP-1 has been identified as a mechanism of glucocorticoid resistance in asthma as AP-1 binds GR and thus prevents its interaction with GRE and other transcription factors (Adcock et al., 1995; Loke et al., 2006). AP-1 is a heterodimer of Fos and Jun proteins and may be activated by pro-inflammatory cytokines such as TNF-α, acting through the JNK pathway. JNK is activated to a greater extent and there is increased expression of c-Fos in PBMC and bronchial biopsies of glucocorticoid-resistant compared with sensitive asthma, with no reduction of JNK activity or c-Jun after high doses of oral glucocorticoids (Lane et al., 1998). This may explain why the increased inflammation found in severe inflammatory disease results in secondary glucocorticoid resistance and is a mechanism for perpetuating resistance whatever the initial mechanism. Increased c-Jun results in depolymerization of the cytoskeleton, which may also reduce GR trans-activating activity (Vardimon et al., 2006). Cofilin-1 is an actin-binding protein that depolymerases the cytoskeleton and in gene array studies was identified as showing increased expression in T cells from glucocorticoid-resistant compared with sensitive asthma (Vasavda et al., 2006). Over-expression of cofilin-1 results in glucocorticoid resistance in T cells.
Abnormal histone acetylation
Histone acetylation plays a critical role in the regulation of inflammatory genes and the mechanism of action of glucocorticoids. Glucocorticoids switch on glucocorticoid-responsive genes, such as MKP-1, via acetylation of specific lysine residues (K5 and K16) on histone-4 (Ito et al., 2000). In a small proportion of patients with glucocorticoid-resistant asthma, GR translocates normally to the nucleus after glucocorticoid exposure but fails to acetylate K5 so that transactivation of genes does not occur (Matthews et al., 2004). These patients show a poor response to high dose inhaled glucocorticoids but unlike most patients with glucocorticoid resistance seem to have fewer side effects as many of these are mediated via GREs (Dostert and Heinzel, 2004).
Recruitment of HDAC2 to activated inflammatory genes is a major mechanism of inflammatory gene repression by glucocorticoids (Barnes et al., 2004) and reduced HDAC2 activity and expression is reduced in some diseases where patients respond poorly. For example, HDAC2 is markedly reduced in alveolar macrophages, airways and peripheral lung in patients with COPD (Ito et al., 2005), and similar changes are found in PBMCs and alveolar macrophages of patients with refractory asthma (Hew et al., 2006) and in the airways of smoking asthmatics (Murahidy et al., 2005). The glucocorticoid resistance of COPD bronchoalveolar macrophages is reversed by over-expressing HDAC2 (using a plasmid vector) to the level seen in control subjects (Ito et al., 2006). The mechanisms for HDAC2 reduction in COPD are now being elucidated (Barnes, 2009b). Oxidative and nitrative stress result in the formation of peroxynitrite, which nitrates tyrosine residues on HDAC2 resulting in its inactivation, ubiquitination and degradation (Ito et al., 2004; Osoata et al., 2009). Oxidative stress also activates phosphoinositide-3-kinase (PI3K)δ, which leads to phosphorylation and inactivation of HDAC2 (Marwick et al., 2010; To et al., 2010). This is confirmed in mice exposed to cigarette smoke that develop glucocorticoid-resistant pulmonary inflammation. This glucocorticoid resistance is completely absent in mice where the PI3Kδ gene is inactivated (Marwick et al., 2009). This suggests that oxidative stress may be an important mechanism of glucocorticoid resistance and is increased in most severe and glucocorticoid-resistant inflammatory diseases.
Decreased regulatory T cells
Interleukin-10 is an important anti-inflammatory and immunoregulatory cytokine and secreted by regulatory T cells in response to glucocorticoids (Hawrylowicz, 2005). In patients with glucocorticoid-resistant asthma there is a failure of T-helper cells to secrete IL-10 but this is restored to normal by vitamin D3 (calcitriol) in vitro (Xystrakis et al., 2006). Furthermore, administration of vitamin D3 to three glucocorticoid-resistant asthmatics also restored the T-cell IL-10 response to glucocorticoids, suggesting that this might be a useful therapeutic approach in the future.
Increased P-glycoprotein
The multidrug resistance gene MDR1 (ABCB1) encodes the drug efflux pump P-glycoprotein 170, a member of the ATP-binding cassette transporters, which transports drugs, including glucocorticoids, out of cells. It has therefore been implicated as a mechanism for glucocorticoid resistance in inflammatory diseases. High levels of expression of MDR1 have been reported in circulating lymphocytes from patients with glucocorticoid-resistant inflammatory bowel disease (Farrell et al., 2000; Farrell and Kelleher, 2003) and rheumatoid arthritis (Tsujimura et al., 2008). Furthermore, certain single nucleotide polymorphisms of MDR1 have been linked to glucocorticoid resistance in these diseases (Potocnik et al., 2004; Drozdzik et al., 2006). However, these observations have not been confirmed in other studies and this mechanism has not been explored in glucocorticoid-resistant airway disease.
Macrophage migration inhibitory factor (MIF)
Macrophage migration inhibitory factor is a pro-inflammatory cytokine that has potent anti-glucocorticoid effects and has been associated with several inflammatory diseases (Flaster et al., 2007). MIF is induced by glucocorticoids and inhibits their anti-inflammatory effects mainly through inhibiting the induction of MKP-1 (Roger et al., 2005). Increased MIF expression has been reported in colonic mononuclear cells from patients with glucocorticoid-resistant ulcerative colitis and a MIF antibody restores the anti-inflammatory response to glucocorticoids in these cells (Ishiguro et al., 2006). Similar findings are reported in glucocorticoid-resistant rheumatoid arthritis and systemic lupus erythematosis. Polymorphisms of the MIF gene have also been reported in association with glucocorticoid resistance (Baugh et al., 2002; Griga et al., 2007), although this is disputed (Ayoub et al., 2008). MIF has also been implicated in the glucocorticoid resistance in asthma (Rossi et al., 1998), suggesting the potential for anti-MIF therapies in glucocorticoid-resistant diseases.
Therapeutic implications
Inhaled glucocorticoids are highly effective in treating most patients with asthma. Patients with severe asthma may require high doses and this has a risk of systemic side effects, which has led to a search for ICS with even greater therapeutic ratios. A few patients with asthma and most patients with COPD are poorly responsive to glucocorticoids and are at risk of side effects, so that alternative anti-inflammatory treatments are needed, or the mechanisms of glucocorticoid resistance need to be reversed. Resistance to the anti-inflammatory effects of glucocorticoids is a major barrier to effective control of many common diseases and enormously increases their morbidity and medical costs.
Dissociated steroids
There has been a concerted effort to develop glucocorticoids that have reduced side effects, while retaining anti-inflammatory efficacy. Selective glucocorticoid receptor agonists (SEGRAs or dissociated steroids) are more effective in trans-repression than trans-activation so have less side effects (Schacke et al., 2007). Several dissociated steroids have now been developed, including non-glucocorticoid GR modulators, but there is uncertainly about the efficacy of these drugs as anti-inflammatory therapies. In a mouse knock-in strain with dimerization-deficient GR some inflammatory processes can be suppressed by glucocorticoids, whereas others can not (Kleiman and Tuckermann, 2007). This may reflect the anti-inflammatory effects of glucocorticoid mediated through transactivation of genes, such as MKP-1. Furthermore, side effects of glucocorticoids may also occur in these mice. While several inhaled non-steroidal GR modulators are currently in clinical development for asthma, there are no studies demonstrating any clinical advantage (De Bosscher, 2010).
Alternative anti-inflammatory treatments
There are several therapeutic strategies to manage glucocorticoid-resistant diseases, but the most important general approaches are to use alternative anti-inflammatory (‘steroid-sparing’) treatments or to reverse the molecular mechanisms of glucocorticoid resistance if these are identified. Several non-steroidal anti-inflammatory drugs are currently available to treat certain glucocorticoid-resistant diseases, but these may have a toxicity of their own. Calcineurin inhibitors, such as cyclosporin A and tacrolimus, may be effective in some patients with glucocorticoid-resistant rheumatoid arthritis, but have not been found to be very effective in glucocorticoid-resistant asthma (Evans et al., 2001; Kitahara and Kawai, 2007). This has led to a search for novel anti-inflammatory treatments, particularly for diseases with marked glucocorticoid resistance, such as COPD, where no effective anti-inflammatory treatments are currently available.
Phosphodiesterase-4 inhibitors are broad spectrum anti-inflammatory treatments that are now in clinical development for several inflammatory diseases, such as COPD (Hatzelmann et al., 2010). However, systemic doses have been limited by side effects, such as nausea, diarrhoea and headaches. Roflumilast is the first PDE4 inhibitors licensed for the treatment of inflammation in COPD patients and reduces neutrophilic inflammation with some improvement in lung function and reduction in exacerbations (Calverley et al., 2009).
Several p38 MAPK inhibitors have been in clinical development and theoretically could be particularly effective in asthma with glucocorticoid resistance due to IL-2 and IL-4, as this is reversed in vitro by selective p38 MAPK inhibitors (Irusen et al., 2002). These drugs may also be useful in other glucocorticoid-insensitive inflammatory diseases such as COPD where p38 MAPK is activated and they have been shown to have efficacy in glucocorticoid-resistant animal models of these diseases (Medicherla et al., 2007). However, these drugs have had problems with toxicity and side effects. Blocking NF-κB by selective inhibitors of inhibitor of NF-κB kinase (IKKβ, IKK2) is another way of treating glucocorticoid-resistant inflammation, but it is likely that these drugs will also have toxicity and side effects so may only be suitable for topical application.
Reversing glucocorticoid resistance
Another therapeutic option for treating glucocorticoid resistance is to reverse the cause of resistance if it can be identified. This is possible with smoking cessation in smoking asthmatics (Chaudhuri et al., 2006) and might be possible for some patients with glucocorticoid-resistant asthma with p38 MAPK, JNK inhibitors and vitamin D3 in the future (Irusen et al., 2002; Loke et al., 2006; Xystrakis et al., 2006). There are several therapeutic strategies for inhibiting P-glycoprotein to prevent the efflux of glucocorticoids, some of which are based on the observations that verapamil and quinidine are efflux blockers; several novel drugs are now in development, but this approach has not been examined in asthma or COPD (Nobili et al., 2006). Increased MIF has been implicated in glucocorticoid resistance in several diseases, so strategies to inhibit MIF, including small molecule inhibitors and monoclonal antibodies, are currently being explored (Hoi et al., 2007).
Selective activation of HDAC2 can be achieved with theophylline, which restores HDAC2 activity in COPD macrophages back to normal and reverses glucocorticoid resistance (Cosio et al., 2004). Mice exposed to cigarette smoke develop glucocorticoid-resistant inflammation which is reversed by low doses of oral theophylline (Fox et al., 2007; To et al., 2010). In COPD patients the combination of theophylline and ICS is more effective in reducing airway inflammation than either drug alone (Ford et al., 2010). This is now leading to therapeutic trials in COPD with low doses of theophylline. Low dose theophylline also improves asthma control in smoking asthmatic patients who show no response to ICS alone (Spears et al., 2009). The molecular mechanism of action of theophylline in restoring HDAC2 appears to be via selective inhibition of PI3Kδ, which is activated by oxidative stress in COPD patients (Marwick et al., 2009; To et al., 2010). This suggests that selective PI3Kδ inhibitors may also be effective and these drugs are currently in clinical development for other diseases. Because oxidative stress appears to be an important mechanism in reducing HDAC2 and leads to glucocorticoid resistance, antioxidants should also be effective. Unfortunately, currently available antioxidants are not very effective and several more potent antioxidants are in clinical development. In the future, novel drugs which increase HDAC2 may be developed when the molecular signalling pathways that regulate HDAC2 are better understood (Barnes, 2005; Barnes, 2009a).
Concluding comments
Glucocorticoids remain by far the most effective therapy for controlling asthma and suppress airway inflammation mainly through repression of activated inflammatory genes, but also by increasing the transcription of anti-inflammatory genes, such as MKP-1. It is unlikely that it will be possible to develop more effective anti-inflammatory treatments for asthma in the future as glucocorticoids have such a broad spectrum of anti-inflammatory actions, reflecting their ability to switch off all activated inflammatory genes. ICS are now amongst the most widely used drugs in the world and there has been considerable effort expended in trying to improve their therapeutic ratio. Addition of long-acting β2-agonists in the form of combination inhalers improves asthma control to a greater extent than increasing the dose of ICS and this has become the standard approach for controlling patients with moderate to severe asthma. This is, at least in part, explained by the favourable molecular interactions between glucocorticoids and β2-agonists. Selective GR modulators which favour trans-repression over trans-activation mechanisms were designed to reduce side effects that are largely due to gene activation, but so far have proved difficult to develop clinically. Furthermore, it is now clear that some anti-inflammatory effects of corticosteroids are due to trans-activation of anti-inflammatory genes, whereas some adverse effects may be due to trans-repression.
The major area of research interest is now focussed on understanding glucocorticoid resistance as it is a major barrier to the effective treatment of COPD patients and asthmatic patients with severe disease or who smoke. The recognition that there are different molecular mechanisms of glucocorticoid resistance has identified several new therapeutic targets. A major mechanism for reduced glucocorticoid responsiveness in COPD, severe and smoking asthma is reduction in HDAC2 activity and expression as a result of oxidative stress via activation of PI3Kδ. This pathway may be blocked by low concentrations of theophylline as well as selective PI3Kδ inhibitors, suggesting new therapeutic approaches to the treatment of severe asthma and COPD in the future. Other drugs that target this pathway are also in development and may lead to a new therapeutic strategy whereby drugs are able to reverse glucocorticoid resistance in airway diseases and perhaps other glucocorticoid-resistant inflammatory diseases, such as atherosclerosis and multiple sclerosis.
Glossary
Abbreviations
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- HDAC
histone deacetylase
- ICS
inhaled corticosteroids
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MKP
MAP kinase phosphatase
- MIF
macrophage migration inhibitory factor
- NF-κB
nuclear factor-κB
- PI3K
phosphoinositide-3-kinase
- TNF
tumour necrosis factor
Conflict of interest
P.B. has received funding for research, lectures and scientific advisory boards from several pharmaceutical companies that are involved in therapy of airway diseases, including AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer, Teva and UCB.
Supporting Information
Teaching Materials; Figs 1–5 as PowerPoint slide.
References
- Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest. 2008;134:394–401. doi: 10.1378/chest.08-0440. [DOI] [PubMed] [Google Scholar]
- Adcock IM, Lane SJ, Brown CA, Lee TH, Barnes PJ. Abnormal glucocorticoid receptor/AP-1 interaction in steroid resistant asthma. J Exp Med. 1995;182:1951–1958. doi: 10.1084/jem.182.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad T, Barnes PJ, Adcock IM. Overcoming steroid insensitivity in smoking asthmatics. Curr Opin Investig Drugs. 2008;9:470–477. [PubMed] [Google Scholar]
- van den Akker EL, Russcher H, van Rossum EF, Brinkmann AO, de Jong FH, Hokken A, et al. Glucocorticoid receptor polymorphism affects transrepression but not transactivation. J Clin Endocrinol Metab. 2006;91:2800–2803. doi: 10.1210/jc.2005-2119. [DOI] [PubMed] [Google Scholar]
- Ayoub S, Hickey MJ, Morand EF. Mechanisms of disease: macrophage migration inhibitory factor in SLE, RA and atherosclerosis. Nat Clin Pract Rheumatol. 2008;4:98–105. doi: 10.1038/ncprheum0701. [DOI] [PubMed] [Google Scholar]
- Baraniuk JN, Ali M, Brody D, Maniscalco J, Gaumond E, Fitzgerald T, et al. Glucocorticoids induce β2-adrenergic receptor function in human nasal mucosa. Am J Respir Crit Care Med. 1997;155:704–710. doi: 10.1164/ajrccm.155.2.9032216. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Scientific rationale for combination inhalers with a long-acting β2-agonists and corticosteroids. Eur Respir J. 2002;19:182–191. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Targeting histone deacetylase 2 in chronic obstructive pulmonary disease treatment. Expert Opin Ther Targets. 2005;9:1111–1121. doi: 10.1517/14728222.9.6.1111. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006a;27:413–426. doi: 10.1183/09031936.06.00125404. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. How corticosteroids control inflammation. Br J Pharmacol. 2006b;148:245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Resp Dis. 2007;29:587–595. doi: 10.1183/09031936.00080306. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Cytokine networks in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008a;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Immunol Rev. 2008b;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Histone deacetylase-2 and airway disease. Ther Adv Respir Dis. 2009a;3:235–243. doi: 10.1177/1753465809348648. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Ann Rev Physiol. 2009b;71:451–464. doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration. 2010a;80:89–95. doi: 10.1159/000315416. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010b;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Ann Intern Med. 2003;139:359–370. doi: 10.7326/0003-4819-139-5_part_1-200309020-00012. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;342:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids: an update. Am J Respir Crit Care Med. 1998;157:S1–S53. doi: 10.1164/ajrccm.157.3.157315. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Ito K, Adcock IM. A mechanism of corticosteroid resistance in COPD: inactivation of histone deacetylase. Lancet. 2004;363:731–733. doi: 10.1016/S0140-6736(04)15650-X. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–563. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, Fitzgerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, et al. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–176. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- Bergmann MW, Staples KJ, Smith SJ, Barnes PJ, Newton R. Glucocorticoid inhibition of GM-CSF from T cells is independent of control by NF-κB and CLE0. Am J Respir Cell Mol Biol. 2004;30:555–563. doi: 10.1165/rcmb.2003-0295OC. [DOI] [PubMed] [Google Scholar]
- Bhavsar P, Hew M, Khorasani N, Alfonso T, Barnes PJ, Adcock I, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared to non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Christodoulopoulos P, Maltais F, Yamauchi Y, Olivenstein R, Hamid Q. Effect of salmeterol/fluticasone propionate on airway inflammation in COPD: a randomised controlled trial. Thorax. 2007;62:938–943. doi: 10.1136/thx.2006.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- Carmichael J, Paterson IC, Diaz P, Crompton GK, Kay AB, Grant IWB. Corticosteroid resistance in asthma. Br Med J. 1981;282:1419–1422. doi: 10.1136/bmj.282.6274.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Livingston E, McMahon AD, Lafferty J, Fraser I, Spears M, et al. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174:127–133. doi: 10.1164/rccm.200510-1589OC. [DOI] [PubMed] [Google Scholar]
- Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? J Endocrinol. 2003;178:5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- Cosio BG, Tsaprouni L, Ito K, Jazrawi E, Adcock IM, Barnes PJ. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med. 2004;200:689–695. doi: 10.1084/jem.20040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt SV, Nightingale JA, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1635–1639. doi: 10.1164/ajrccm.160.5.9811058. [DOI] [PubMed] [Google Scholar]
- De Bosscher K. Selective Glucocorticoid Receptor modulators. J Steroid Biochem Mol Biol. 2010;120:96–104. doi: 10.1016/j.jsbmb.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Donn R, Berry A, Stevens A, Farrow S, Betts J, Stevens R, et al. Use of gene expression profiling to identify a novel glucocorticoid sensitivity determining gene, BMPRII. FASEB J. 2007;21:402–414. doi: 10.1096/fj.06-7236com. [DOI] [PubMed] [Google Scholar]
- Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10:2807–2816. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- Drozdzik M, Rudas T, Pawlik A, Kurzawski M, Czerny B, Gornik W, et al. The effect of 3435C>T MDR1 gene polymorphism on rheumatoid arthritis treatment with disease-modifying antirheumatic drugs. Eur J Clin Pharmacol. 2006;62:933–937. doi: 10.1007/s00228-006-0192-1. [DOI] [PubMed] [Google Scholar]
- Erin EM, Zacharasiewicz AS, Nicholson GC, Tan AJ, Neighbour H, Engelstatter R, et al. Rapid anti-inflammatory effect of inhaled ciclesonide in asthma: a randomised, placebo-controlled study. Chest. 2008;134:740–745. doi: 10.1378/chest.07-2575. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Cullinan P, Geddes DM. Cyclosporin as an oral corticosteroid sparing agent in stable asthma (Cochrane Review) Cochrane Database Syst Rev. 2001;2:CD002993. doi: 10.1002/14651858.CD002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhri S, Tulic M, Christodoulopoulos P, Fukakusa M, Frenkiel S, Leung DY, et al. Microbial superantigens induce glucocorticoid receptor beta and steroid resistance in a nasal explant model. Laryngoscope. 2004;114:887–892. doi: 10.1097/00005537-200405000-00019. [DOI] [PubMed] [Google Scholar]
- Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol. 2003;178:339–346. doi: 10.1677/joe.0.1780339. [DOI] [PubMed] [Google Scholar]
- Farrell RJ, Murphy A, Long A, Donnelly S, Cherikuri A, O'Toole D, et al. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology. 2000;118:279–288. doi: 10.1016/s0016-5085(00)70210-1. [DOI] [PubMed] [Google Scholar]
- Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinol. 2007;21:1267–1280. doi: 10.1210/me.2007-0065. [DOI] [PubMed] [Google Scholar]
- Ford PA, Durham AL, Russell REK, Gordon F, Adcock IM, Barnes PJ. Treatment effects of low dose theophylline combined with an inhaled corticosteroid in COPD. Chest. 2010;137:1338–1344. doi: 10.1378/chest.09-2363. [DOI] [PubMed] [Google Scholar]
- Fox JC, Spicer D, Ito K, Barnes PJ, Fitzgerald MF. Oral or inhaled corticosteroid combination therapy with low dose theophylline reverses corticosteroid insensitivity in a smoking mouse model. Proc Am Thorac Soc. 2007;2:A637. [Google Scholar]
- Gagliardo R, Chanez P, Vignola AM, Bousquet J, Vachier I, Godard P, et al. Glucocorticoid receptor α and β in glucocorticoid dependent asthma. Am J Respir Crit Care Med. 2000;162:7–13. doi: 10.1164/ajrccm.162.1.9911032. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Piwien-Pilipuk G, Assreuy J. Inhibition of glucocorticoid receptor binding by nitric oxide. Mol Pharmacol. 1999;55:317–323. doi: 10.1124/mol.55.2.317. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Saltos N, Fakes K. Acute anti-inflammatory effects of inhaled budesonide in asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2001;163:32–36. doi: 10.1164/ajrccm.163.1.9807061. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Powell H, Ducharme FM. Differential effects of maintenance long-acting beta-agonist and inhaled corticosteroid on asthma control and asthma exacerbations. J Allergy Clin Immunol. 2007;119:344–350. doi: 10.1016/j.jaci.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Giembycz MA, Kaur M, Leigh R, Newton R. A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br J Pharmacol. 2008;153:1090–1104. doi: 10.1038/sj.bjp.0707627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol. 2002;169:5934–5940. doi: 10.4049/jimmunol.169.10.5934. [DOI] [PubMed] [Google Scholar]
- Goleva E, Li LB, Eves PT, Strand MJ, Martin RJ, Leung DY. Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 2006;173:607–616. doi: 10.1164/rccm.200507-1046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griga T, Wilkens C, Wirkus N, Epplen J, Schmiegel W, Klein W. A polymorphism in the macrophage migration inhibitory factor gene is involved in the genetic predisposition of Crohn's disease and associated with cumulative steroid doses. Hepatogastroenterology. 2007;54:784–786. [PubMed] [Google Scholar]
- Hakonarson H, Bjornsdottir US, Halapi E, Bradfield J, Zink F, Mouy M, et al. Profiling of genes expressed in peripheral blood mononuclear cells predicts glucocorticoid sensitivity in asthma patients. Proc Natl Acad Sci U S A. 2005;102:14789–14794. doi: 10.1073/pnas.0409904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, et al. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 1999;159:1600–1604. doi: 10.1164/ajrccm.159.5.9804131. [DOI] [PubMed] [Google Scholar]
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165:1592–1596. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, et al. The preclinical pharmacology of roflumilast – a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23:235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med. 2005;202:1459–1463. doi: 10.1084/jem.20052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew M, Bhavsar P, Torrego A, Meah S, Khorasani N, Barnes PJ, et al. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med. 2006;174:134–141. doi: 10.1164/rccm.200512-1930OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi AY, Iskander MN, Morand EF. Macrophage migration inhibitory factor: a therapeutic target across inflammatory diseases. Inflamm Allergy Drug Targets. 2007;6:183–190. doi: 10.2174/187152807781696455. [DOI] [PubMed] [Google Scholar]
- Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: Role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109:649–657. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- Ishiguro Y, Ohkawara T, Sakuraba H, Yamagata K, Hiraga H, Yamaguchi S, et al. Macrophage migration inhibitory factor has a proinflammatory activity via the p38 pathway in glucocorticoid-resistant ulcerative colitis. Clin Immunol. 2006;120:335–341. doi: 10.1016/j.clim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits IL-1β-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Tomita T, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase (HDAC)2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Sabo PJ, Johnson TA, Sung MH, Biddie SC, Lightman SL, et al. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell. 2008;29:611–624. doi: 10.1016/j.molcel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Kline PA, Yan Zieleshem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Long-term effects of budesonide on airway responsiveness and clinical asthma severity in inhaled steroid-dependent asthmatics. Eur Respir J. 1990;3:1122–1127. [PubMed] [Google Scholar]
- Keatings VM, Jatakanon A, Worsdell YM, Barnes PJ. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med. 1997;155:542–548. doi: 10.1164/ajrccm.155.2.9032192. [DOI] [PubMed] [Google Scholar]
- Ketchell RI, Jensen MW, Lumley P, Wright AM, Allenby MI, O'Connor BJ. Rapid effect of inhaled fluticasone propionate on airway responsiveness to adenosine 5'-monophosphate in mild asthma. J Allergy Clin Immunol. 2002;110:603–606. doi: 10.1067/mai.2002.128486. [DOI] [PubMed] [Google Scholar]
- Kitahara K, Kawai S. Cyclosporine and tacrolimus for the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:238–245. doi: 10.1097/BOR.0b013e328099af80. [DOI] [PubMed] [Google Scholar]
- Kleiman A, Tuckermann JP. Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol Cell Endocrinol. 2007;275:98–108. doi: 10.1016/j.mce.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Kozaci DL, Chernajovsky Y, Chikanza IC. The differential expression of corticosteroid receptor isoforms in corticosteroid-resistant and -sensitive patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:579–585. doi: 10.1093/rheumatology/kel276. [DOI] [PubMed] [Google Scholar]
- Lamberts SW. Hereditary glucocorticoid resistance. Ann Endocrinol (Paris) 2001;62:164–167. [PubMed] [Google Scholar]
- Lane SJ, Arm JP, Staynov DZ, Lee TH. Chemical mutational analysis of the human glucocortiocoid receptor cDNA in glucocorticoid-resistant bronchial asthma. Am J Respir Cell Mol Biol. 1994;11:42–48. doi: 10.1165/ajrcmb.11.1.8018337. [DOI] [PubMed] [Google Scholar]
- Lane SJ, Adcock IM, Richards D, Hawrylowicz C, Barnes PJ, Lee TH. Corticosteroid-resistant bronchial asthma is associated with increased c-Fos expression in monocytes and T-lymphocytes. J Clin Invest. 1998;102:2156–2164. doi: 10.1172/JCI2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DYM, Martin RJ, Szefler SJ, Sher ER, Ying S, Kay AB, et al. Dysregulation of interleukin 4, interleukin 5, and interferon y gene expression in steroid-resistant asthma. J Exp Med. 1995;181:33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Cidlowski JA. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann N Y Acad Sci. 2006;1069:1–9. doi: 10.1196/annals.1351.001. [DOI] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, Goleva E, Hall CF, Ou LS, Leung DY. Superantigen-induced corticosteroid resistance of human T cells occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK-ERK) pathway. J Allergy Clin Immunol. 2004;114:1059–1069. doi: 10.1016/j.jaci.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Loke TK, Mallett KH, Ratoff J, O'Connor BJ, Ying S, Meng Q, et al. Systemic glucocorticoid reduces bronchial mucosal activation of activator protein 1 components in glucocorticoid-sensitive but not glucocorticoid-resistant asthmatic patients. J Allergy Clin Immunol. 2006;118:368–375. doi: 10.1016/j.jaci.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Loppow D, Schleiss MB, Kanniess F, Taube C, Jorres RA, Magnussen H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respir Med. 2001;95:115–121. doi: 10.1053/rmed.2000.0960. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. The origin and functions of multiple human glucocorticoid receptor isoforms. Ann N Y Acad Sci. 2004;1024:102–123. doi: 10.1196/annals.1321.008. [DOI] [PubMed] [Google Scholar]
- Mak JC, Chuang TT, Harris CA, Barnes PJ. Increased expression of G protein-coupled receptor kinases in cystic fibrosis lung. Eur J Pharmacol. 2002;436:165–172. doi: 10.1016/s0014-2999(01)01625-9. [DOI] [PubMed] [Google Scholar]
- Mak JCW, Nishikawa M, Barnes PJ. Glucocorticosteroids increase β2-adrenergic receptor transcription in human lung. Am J Physiol. 1995a;12:L41–L46. doi: 10.1152/ajplung.1995.268.1.L41. [DOI] [PubMed] [Google Scholar]
- Mak JCW, Nishikawa M, Shirasaki H, Miyayasu K, Barnes PJ. Protective effects of a glucocorticoid on down-regulation of pulmonary β2-adrenergic receptors in vivo. J Clin Invest. 1995b;96:99–106. doi: 10.1172/JCI118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneechotesuwan K, Xin Y, Ito K, Jazrawi E, Lee KY, Usmani OS, et al. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J Immunol. 2007;178:2491–2498. doi: 10.4049/jimmunol.178.4.2491. [DOI] [PubMed] [Google Scholar]
- Maneechotesuwan K, Supawita S, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Sputum indoleamine-2, 3-dioxygenase activity is increased in asthmatic airways by using inhaled corticosteroids. J Allergy Clin Immunol. 2008;121:43–50. doi: 10.1016/j.jaci.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Maneechotesuwan K, Yao X, Ito K, Jazrawi E, Usmani OS, Adcock IM, et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 2009;6:e1000076. doi: 10.1371/journal.pmed.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol. 2010;126:754–762. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Marwick JA, Caramori G, Stevenson CC, Casolari P, Jazrawi E, Barnes PJ, et al. Inhibition of PI3Kδ restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med. 2009;179:542–548. doi: 10.1164/rccm.200810-1570OC. [DOI] [PubMed] [Google Scholar]
- Marwick JA, Caramori G, Casolari P, Mazzoni F, Kirkham PA, Adcock IM, et al. A role for phosphoinositol 3-kinase delta in the impairment of glucocorticoid responsiveness in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2010;125:1146–1153. doi: 10.1016/j.jaci.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–1108. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Medicherla S, Fitzgerald M, Spicer D, Woodman P, Ma JY, Kapoun AM, et al. p38α selective MAP kinase inhibitor, SD-282, reduces inflammation in a sub-chronic model of tobacco smoke-induced airway inflammation. J Pharmacol Exp Ther. 2007;324:921–929. doi: 10.1124/jpet.107.127092. [DOI] [PubMed] [Google Scholar]
- Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, et al. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–1583. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- Murahidy A, Ito M, Adcock IM, Barnes PJ, Ito K. Reduction is histone deacetylase expression and activity in smoking asthmatics: a mechanism of steroid resistance. Proc Am Thorac Soc. 2005;2:A889. [Google Scholar]
- Newton R, Leigh R, Giembycz MA. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacol Ther. 2010;125:286–327. doi: 10.1016/j.pharmthera.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili S, Landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7:861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Effects of early intervention with inhaled budesonide on lung function in newly diagnosed asthma. Chest. 2006;129:1478–1485. doi: 10.1378/chest.129.6.1478. [DOI] [PubMed] [Google Scholar]
- Orii F, Ashida T, Nomura M, Maemoto A, Fujiki T, Ayabe T, et al. Quantitative analysis for human glucocorticoid receptor alpha/beta mRNA in IBD. Biochem Biophys Res Commun. 2002;296:1286–1294. doi: 10.1016/s0006-291x(02)02030-2. [DOI] [PubMed] [Google Scholar]
- Osoata G, Yamamura S, Ito M, Vuppusetty C, Adcock IM, Barnes PJ, et al. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem Biophys Res Commun. 2009;384:366–371. doi: 10.1016/j.bbrc.2009.04.128. [DOI] [PubMed] [Google Scholar]
- Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun. 2004;5:530–539. doi: 10.1038/sj.gene.6364123. [DOI] [PubMed] [Google Scholar]
- Pujols L, Mullol J, Picado C. Alpha and beta glucocorticoid receptors: relevance in airway diseases. Curr Allergy Asthma Rep. 2007;7:93–99. doi: 10.1007/s11882-007-0005-3. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of COPD – 2006 Update. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Roger T, Chanson AL, Knaup-Reymond M, Calandra T. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol. 2005;35:3405–3413. doi: 10.1002/eji.200535413. [DOI] [PubMed] [Google Scholar]
- Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, et al. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Invest. 1998;101:2869–2874. doi: 10.1172/JCI1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Johnson PR, Rudiger JJ, King GG, Ge Q, Burgess JK, et al. Interaction between glucocorticoids and β2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–1299. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- Schacke H, Berger M, Rehwinkel H, Asadullah K. Selective glucocorticoid receptor agonists (SEGRAs): novel ligands with an improved therapeutic index. Mol Cell Endocrinol. 2007;275:109–117. doi: 10.1016/j.mce.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Sher ER, Leung YM, Surs W, Kam JC, Zieg G, Kamada AK, et al. Steroid-resistant asthma. Cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest. 1994;93:33–39. doi: 10.1172/JCI116963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoak K, Cidlowski JA. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol. 2006;26:9126–9135. doi: 10.1128/MCB.00679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AR, Lane SJ, Cidlowski JA, Staynov DZ, Lee TH. Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol. 2000;105:943–950. doi: 10.1067/mai.2000.106486. [DOI] [PubMed] [Google Scholar]
- Spahn JD, Szefler SJ, Surs W, Doherty DE, Nimmagadda SR, Leung DY. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol. 1996;157:2654–2659. [PubMed] [Google Scholar]
- Spears M, Donnelly I, Jolly L, Brannigan M, Ito K, McSharry C, et al. Effect of theophylline plus beclometasone on lung function in smokers with asthma-a pilot study. Eur Respir J. 2009;33:1010–1017. doi: 10.1183/09031936.00158208. [DOI] [PubMed] [Google Scholar]
- Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J. 2009;34:13–16. doi: 10.1183/09031936.00190908. [DOI] [PubMed] [Google Scholar]
- Szatmary Z, Garabedian MJ, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem. 2004;279:43708–43715. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- Tao T, Lan J, Lukacs GL, Hache RJ, Kaplan F. Importin 13 regulates nuclear import of the glucocorticoid receptor in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;35:668–680. doi: 10.1165/rcmb.2006-0073OC. [DOI] [PubMed] [Google Scholar]
- Thomson NC, Spears M. The influence of smoking on the treatment response in patients with asthma. Curr Opin Allergy Clin Immunol. 2005;5:57–63. doi: 10.1097/00130832-200502000-00011. [DOI] [PubMed] [Google Scholar]
- To M, Ito K, Kizawa Y, Failla M, Ito M, Kusama T, et al. Targeting phosphoinositide-3-kinase-δ with theophylline reverses corticosteroid insensitivity in COPD. Am J Respir Crit Care Med. 2010;182:897–904. doi: 10.1164/rccm.200906-0937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura S, Saito K, Nawata M, Nakayamada S, Tanaka Y. Overcoming drug resistance induced by P-glycoprotein on lymphocytes in patients with refractory rheumatoid arthritis. Ann Rheum Dis. 2008;67:380–388. doi: 10.1136/ard.2007.070821. [DOI] [PubMed] [Google Scholar]
- Usmani OS, Ito K, Maneechotesuwan K, Ito M, Johnson M, Barnes PJ, et al. Glucocorticoid receptor nuclear translocation in airway cells following inhaled combination therapy. Am J Respir Crit Care Med. 2005;172:704–712. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- Vardimon L, Ben Dror I, Oren A, Polak P. Cytoskeletal and cell contact control of the glucocorticoid pathway. Mol Cell Endocrinol. 2006;252:142–147. doi: 10.1016/j.mce.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Vasavda N, Eichholtz T, Takahashi A, Affleck K, Matthews JG, Barnes PJ, et al. Expression of nonmuscle cofilin-1 and steroid responsiveness in severe asthma. J Allergy Clin Immunol. 2006;118:1090–1096. doi: 10.1016/j.jaci.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Wallace AD, Cidlowski JA. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J Biol Chem. 2001;276:42714–42721. doi: 10.1074/jbc.M106033200. [DOI] [PubMed] [Google Scholar]
- Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol. 2007;21:2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IA, Fong KM, Sim EH, Black PN, Lasserson TJ. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007:CD002991. doi: 10.1002/14651858.CD002991.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


