Abstract
Background
Currently, over 340,000 individuals are receiving long-term hemodialysis (HD) therapy for end-stage renal disease and therefore are particularly vulnerable to influenza, prone to more severe influenza outcomes, and less likely to achieve seroprotection from standard influenza vaccines. Influenza vaccine adjuvants, chemical or biological compounds added to a vaccine to boost the elicited immunological response, may help overcome this problem.
Study design
Economic stochastic decision analytic simulation model.
Setting & Participants
United States adult HD population.
Model, Perspective, & Timeframe
The model simulated the decision to use either an adjuvanted or non-adjuvanted vaccine, assumed the societal perspective, and represented a single influenza season, or 1 year.
Intervention
Adjuvanted influenza vaccine at different adjuvant costs and efficacies. Sensitivity analyses explored the impact of varying the influenza clinical attack rate, influenza hospitalization rate, and influenza-related mortality.
Outcomes
Incremental cost-effectiveness ratio (ICER) of adjuvanted influenza vaccine (versus non-adjuvanted) with effectiveness measured in quality-adjusted life-years (QALYs).
Results
Adjuvanted influenza vaccine would be cost-effective (ICER<$50,000/QALY) at a $1 adjuvant cost (on top of the standard vaccine cost) when the adjuvant efficacy (in overcoming the difference between influenza vaccine response in HD patients and healthy adults) ≥60% and economically dominant (provides both cost savings and health benefits) when the $1 adjuvant's efficacy is 100%. A $2 adjuvant would be cost-effective should the adjuvant efficacy be 100%.
Limitations
All models are simplifications of real life and cannot possibly capture all possible factors and outcomes.
Conclusions
An adjuvanted influenza vaccine with adjuvant cost ≤$2 could be cost-effective strategy in a standard influenza season depending on the potency of the adjuvant.
Index Words: Influenza Vaccine, Hemodialysis, Vaccine Adjuvant, Seasonal Influenza, Computer Simulation, Computer model, Cost-effectiveness, Immunodeficiency, End-Stage Renal Disease
Currently, over 340,000 individuals are receiving long-term hemodialysis (HD) therapy for end-stage renal disease and therefore are particularly vulnerable to influenza, prone to more severe influenza outcomes, and less likely to achieve seroprotection from standard influenza vaccines1-10. Influenza vaccine adjuvants, chemical or biological compounds added to a vaccine to boost the elicited immunological response, may help overcome this problem. Adjuvanted influenza vaccines are currently on the market in Europe and in late clinical development in the United States for the general older adult population, who demonstrate decreased responses to non-adjuvanted vaccines due to immunosenescence11-18. The HD population is a potential target for such adjuvants as well17 and, in fact, have been the subjects of recent clinical trials18.
While a prior study explored the potential economic value of an adjuvanted influenza vaccine in the older adult population, the economic value of an adjuvanted vaccine in the HD population remains unclear19. Therefore, we developed a computer simulation model to estimate the potential economic value of a seasonal influenza vaccine adjuvant for adults receiving regular HD. Sensitivity analyses explored the effect of varying the adjuvant cost and efficacy, influenza risk, and probabilities of various influenza outcomes.
Methods
Decision Model
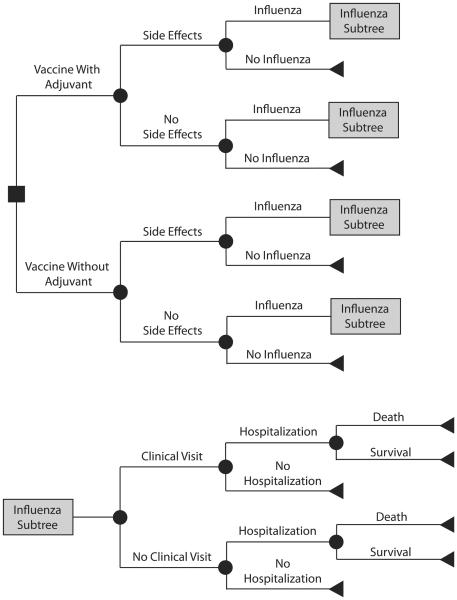
Figure 1 outlines the general structure of the computational decision analytic model, developed using TreeAge Pro 2009 (TreeAge Software, www.treeage.com), which simulated the decision between using an adjuvanted versus a non-adjuvanted influenza vaccine in an adult patient (median age: 64 years old) requiring chronic HD10. The model assessed the cost-effectiveness of this decision from the societal perspective. Each vaccinated patient had a risk of vaccine side effects (i.e., local pain or inflammation), which would require over-the-counter anti-inflammatory medications. Each individual receiving the non-adjuvanted vaccine had a risk of contracting influenza, determined by the seasonal influenza attack rate mitigated by the efficacies of the vaccine. The adjuvant gap bridged the gap between influenza vaccine efficacy in a HD patient and a healthy adult by a proportion (i.e., adjuvant efficacy).
Figure 1.
General model structure
Vaccine efficacy reduces the individual's risk of influenza by 1 – vaccine efficacy and, if the individual contracts influenza, the risk of hospitalization and mortality by 1 – vaccine efficacy (i.e., a 100% efficacious vaccine would reduce a patient's probability of getting influenza to zero; a 75% efficacious vaccine would reduce a patient's probabilities of developing influenza, being hospitalized if he or she develops influenza, and not surviving influenza by 75%). Adjuvant efficacy is the degree to which the adjuvant increases influenza vaccine efficacy from observed levels in HD patients to those of healthy adults. So, a 100% efficacious adjuvant brings influenza vaccine efficacy in a HD patient (e.g., 63%) to levels seen in healthy adults (e.g., 80%). A 75% efficacious adjuvant covers the gap between influenza vaccine efficacy in a HD patient (e.g., 63%) and that of a health adult by 75% (e.g., 63% to 72%). 100% adjuvant efficacy means that a HD patient had influenza vaccine efficacy equal to that of a healthy adult. In other words, each individual receiving the adjuvanted vaccine had a risk of developing influenza corresponding to PNAV-HD + [Adjuvant Efficacy × (PNAV − PNAV )], where PNAV-HD is the probability of an HD patient developing influenza after receiving a nonadjuvanted vaccine and PNAV is the probability of a healthy adult developing influenza after receiving a nonadjuvanted vaccine.
Contracting influenza could result in asymptomatic infection or symptomatic infection followed by a visit to the clinic, hospitalization, or death.
Data Inputs
Table 1 lists the data inputs for our model along with distributions and sources. Where possible, data came from published meta-analyses. Probability and utility variables drew from beta distributions while cost and duration variables drew from gamma distributions. Variables which had limited data drew from triangular distributions. A 3% discount rate adjusted all costs to 2010 US dollars20.
Table 1.
Model Inputs
| Description (units) | Mean | Lower Limit1 |
Upper Limit1 |
Distribution1 | Source |
|---|---|---|---|---|---|
| Costs ($U.S.) | |||||
| Standard Influenza Vaccine | 16.22 | 12.16 | 20.28 | Triangular | Redbook 49 |
| Influenza treatment | |||||
| OTC medications | 16.08 | 12.05 | 20.10 | Triangular | Redbook 49 |
| Outpatient visit given influenza | 104.77 | 78.58 | 130.96 | Triangular | CMS50 |
| Productivity loss for outpatient visit | 66.00 | 60.81 | 71.15 | Triangular | U.S. Department of Labor 51 |
| Hospitalization | 6260.08 | 6102.33 | 6417.74 | Gamma | www.hcup-us.ahrq.gov/reports 52 |
| Death in hospital | 5150.00 | 3862.50 | 6437.50 | Triangular | Smith53 |
| Treatment of vaccine side effects | |||||
| OTC medications | 0.78 | 0.70 | 3.93 | Triangular | Redbook 49 |
| Durations | |||||
| Influenza (days) | 7 | 5.25 | 8.75 | Gamma | MMWR; Widquist; Gubareva; Hayden; Jefferson; Treanor54-59 |
| Life expectancy for 64 year old patient on HD (years)2 |
4.8 | -- | -- | -- | USRDS10 |
| Utilities (QALYs) | |||||
| One year of life for HD patient | 0.49 | 0.45 | 0.53 | Beta | Tengs60 |
| Vaccine Side effects | 0.95 | 0.71 | 1 | Triangular | Tengs60 |
| Influenza with hospitalization | 0.5 | 0.38 | 0.63 | Triangular | Tengs; Sackett60-61 |
| Influenza without hospitalization | 0.65 | 0.49 | 0.81 | Triangular | Tengs; Sackett 60-61 |
| Probabilities | |||||
| Influenza without vaccination | 0.125 | 0.05 | 0.2 | Triangular | Rivetti 62 |
| Outpatient visit if develop influenza | 0.625 | 0.507 | 0.743 | Triangular | Molinari 26 |
| Hospitalization if develop influenza (diabetes patient)2 | 0.012 | -- | -- | -- | Looijmans-Van den Akker 63 |
| Death in hospital given influenza (diabetes patient)2 | 0.002 | -- | -- | -- | Looijmans-Van den Akker 63 |
| Vaccine without adjuvant | |||||
| Side effects | 0.63 | 0.47 | 0.89 | Triangular | Langley; Roman; Clark 40, 42, 64 |
| Vaccine efficacy (healthy adults) | 0.8 | 0.56 | 0.91 | Triangular | Demicheli 65 |
| Vaccine efficacy (adult HD patients) | 0.63 | 0.59 | 0.69 | Triangular | Scharpe21 |
| Vaccine with adjuvant | |||||
| Side effects | 0.48 | 0.39 | 0.59 | Triangular | Langley; Roman; Clark 40, 42, 64 |
Dashes indicate that point estimates were used for these parameters.
Variables were based on single values due to lack of available data in the literature. Sensitivity analyses were run to account for error and uncertainty.
CMS, Centers for Medicare and Medicaid Services; USRDS, US Renal Data System; OTC, over-the-counter; HD, hemodialysis; QALY, quality-adjusted life-year
Limited available data on influenza clinical attack rates in the hemodialysis population required us to use serological data from clinical studies that reported seroprotection rates. The definition of seroprotection is a hemagglutination inhibition (HI) antibody titer ≥40 21. Where hospitalization and mortality data for HD patients was not available, data from diabetic populations, who have similar influenza outcomes, served as a proxy22-24. Sensitivity analyses analyzed the robustness of this assumption.
Each simulation run consisted of sending 1000 hypothetical adult HD patients (median age: 64 years old) through the model 1000 times for a total of 1,000,000 trials. For each run, the incremental cost-effectiveness ratio (ICER) of the adjuvanted vaccine versus the standard vaccine was calculated as the ratio of cost difference between the adjuvanted and nonadjuvanted vaccines to the difference in effectiveness of the adjuvanted and nonadjuvanted vaccines.
The measure of effectiveness measured was quality-adjusted life years (QALYs). Adjuvanted vaccine was considered cost-effective if the ICER fell below $50,000/QALY, an often cited threshold 25.
Sensitivity Analyses
Sensitivity analyses explored the impact of varying key variables, including adjuvant efficacy (range: 0-100%), adjuvant cost, i.e., the added cost of an adjuvant to the influenza vaccination cost (range: $0-$5), probability of clinic visit when sick with influenza (range: 0-80%), probability of hospitalization from influenza (range: 0.25x-10x baseline of 1.2%, and mortality (range: 0.25x-10x baseline of 0.2%) from influenza. Additional analyses also explored the impact of requiring a second administration of the adjuvanted vaccine. Each dose had a given efficacy and cost. An individual had a treatment adherence value reflecting whether the second dose was received (e.g., 100% adherence meant that each individual received the second dose; 75% mean that only three-quarters of individuals received the second dose). Each dose had a risk of side effects. Additionally, probabilistic sensitivity analyses determined the effects of simultaneously varying all variables across the distributions listed in Table 2.
Table 2.
Outline of Sensitivity Analyses
| Model Parameter | Sensitivity Analysis Range |
|---|---|
| Adjuvant Cost | $1-$5 |
| Adjuvant Efficacy† | 0-100% |
| Annual Influenza Probability | 5-20% |
| Doses of Adjuvanted Vaccine Required | 1-2 doses |
| Patient Treatment Adherence for Second Dose of Adjuvanted Vaccine |
25-100% |
| Probability of a Clinic Visit for Influenza†† | 0-80% |
| Probability of Hospitalization for Influenza†† | 0.3-12.0% |
| Probability of Influenza Attributable Mortality†† | 0.05-2.0% |
Vaccine efficacy reduces the individual's risk of influenza by 1 – vaccine efficacy and, if the individual contracts influenza, the risk of hospitalization and mortality by 1 – vaccine efficacy (i.e., a 100% efficacious vaccine would reduce a patient's probability of getting influenza to zero; a 75% efficacious vaccine would reduce a patient's probabilities of developing influenza, being hospitalized if he or she develops influenza, and not surviving influenza by 25%).
Adjuvant efficacy is the degree to which the adjuvant increases influenza vaccine efficacy from observed levels in HD patients to those of healthy adults. So, a 100% efficacious adjuvant brings influenza vaccine efficacy in a HD patient (e.g., 63%) to levels seen in healthy adults (e.g., 80%). A 75% efficacious adjuvant covers the gap between influenza vaccine efficacy in a HD patient (e.g., 63%) and that of a health adult by 75% (e.g., 63% to 72%).
Results
Overall Results
Table 3 shows how the ICER of employing an adjuvanted vaccine versus nonadjuvanted varies with adjuvant cost and efficacy and clinical influenza attack rate. The ICER is fairly sensitive to the adjuvant cost. In general, the adjuvanted vaccine is no longer cost-effective (i.e., ICER >$50,000/QALY) when adjuvant cost exceeds $2. The adjuvant efficacy also drives the ICER. The adjuvant efficacy should be at least 60% for the ICER to be below $50,000/QALY. A $1 adjuvant with 100% efficacy, i.e., can make a vaccine induce an immune response in an HD patient equivalent to that in a healthy adult, will actually be economically dominant (i.e., provide both cost-savings and health benefits).
Table 3.
ICER of Adjuvanted versus Non-Adjuvanted Vaccine
| Adjuvant Efficacy |
Influenza Clinical Attack Rate | ||||
|---|---|---|---|---|---|
| Base Case‡ | 12.50% | 15.00% | 17.50% | 20.00% | |
| Adjuvant Cost=$1 | |||||
| 20% | 208,861 | 248,331 | 58,642 | 16,390 | Dominated* |
| 40% | 85,323 | 108,499 | Dominated* | Dominated* | Dominated* |
| 60% | 32,433 | 20,185 | Dominated* | Dominated* | Dominated* |
| 80% | 3,275 | 12,249 | Dominated* | Dominated* | Dominated* |
| 100%† | Dominated* | Dominated* | Dominated* | Dominated* | Dominated* |
| Adjuvant Cost=$2 | |||||
| 20% | 525,475 | 419,332 | 153,765 | 51,397 | Dominated* |
| 40% | 193,703 | 122,258 | 33,166 | 1,061 | Dominated* |
| 60% | 99,801 | 70,803 | Dominated* | Dominated* | Dominated* |
| 80% | 53,634 | 37,536 | Dominated* | Dominated* | Dominated* |
| 100%† | 38,357 | 16,851 | Dominated* | Dominated* | Dominated* |
| Adjuvant Cost=$5 | |||||
| 20% | 1,128,830 | 1,087,534 | 439,505 | 249,265 | 32,665 |
| 40% | 525,173 | 458,354 | 154,171 | 93,731 | Dominated* |
| 60% | 227,850 | 361,572 | 102,060 | 37,879 | Dominated* |
| 80% | 241,426 | 226,823 | 49,969 | 13,647 | Dominated* |
| 100%† | 192,097 | 185,804 | 26,340 | Dominated* | Dominated* |
Values are presented ICERs with units of $/QALY. Shaded values indicate cases where the adjuvanted strategy is cost-effective.
Adjuvanted vaccine strategy dominates the non-adjuvanted vaccine strategy (i.e. less costly and more effective)
100% adjuvant efficacy denotes influenza vaccine efficacy equal to that of a healthy adult.
The base case clinical influenza attack rate draws from a triangular distribution, with mean: 0.125, range: 0.05-0.20.
ICER, Incremental Cost-Effectiveness Ratio
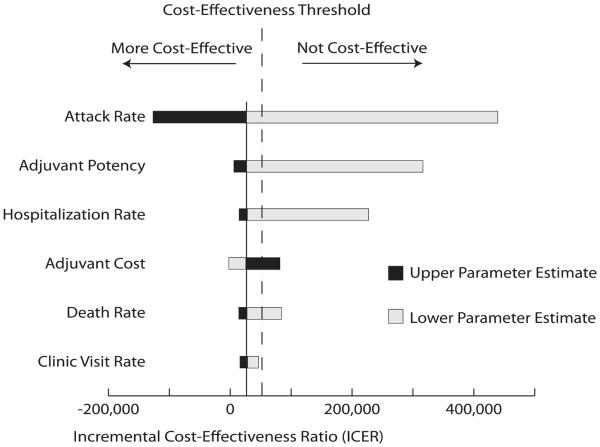
Figure 2 is a tornado diagram where each bar represents a one-way sensitivity analysis to visually compare the impact of changing each parameter's value on the incremental cost-effectiveness of adding adjuvant to the influenza vaccine. The diagram arranges parameters from top to bottom in the order of their impact on the ICER values. The dark bars indicate when the parameter value is the upper half of its range, whereas the light bars indicate when it is in the lower half. For example, as the attack rate and adjuvant potency increase, the ICER decreases. Conversely, decreasing adjuvant cost decreases the ICER value. The solid vertical line on the diagram marks the ICER when all parameters are set to their baseline values. The dotted line delineates where the ICER equals $50,000/QALY so that horizontal bars to the left of this line indicate where adding adjuvant is cost-effective, bars to the right is where adding adjuvant is not. This figure highlights that the attack rate and adjuvant potency have the greatest relative impact on the cost-effectiveness of adding adjuvant, whereas influenza mortality and probability of a separate clinical visit for influenza have the least.
Figure 2.
Tornado Diagram
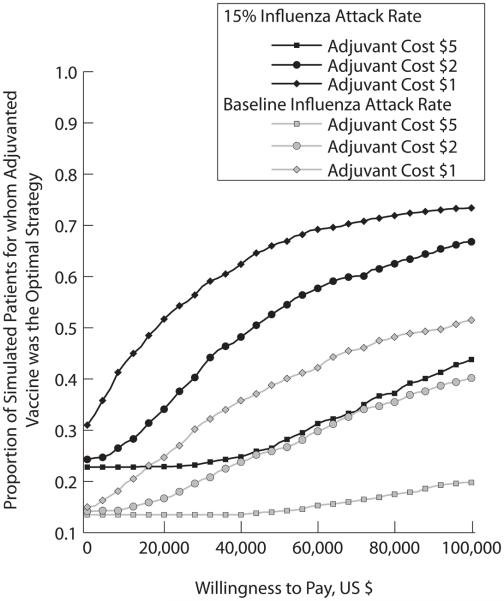
ICER values show that in a standard influenza season, an adjuvant can be cost-effective at $1 and $2 premiums to the cost of the seasonal influenza vaccine given the adjuvant's efficacy, as shown by the base case values in Table 3. Trends show that when the adjuvant costs more than $2, it ceases to be a cost-effective strategy. Figure 3 shows acceptability curves for 60% adjuvant potency for varying adjuvant cost and influenza attack rates. Decreasing adjuvant cost increases the proportion of simulated patients for whom adjuvanted vaccine was the optimal strategy. On the other hand, increasing influenza attack rate increases the proportion of simulated patients for whom adjuvanted vaccination was economically favorable over non-adjuvanted vaccination.
Figure 3.
Acceptability Curves for 60% Adjuvant Potency for Varying Adjuvant Cost and Influenza Attack Rates
Table 3 shows that ICER is fairly sensitive to the influenza clinical attack rate. A worse than usual (either one with a higher symptomatic rate or higher overall attack rate) influenza season or epidemic would make the adjuvanted vaccine at lower adjuvant efficacies and higher adjuvant costs. For example, if a $1 adjuvant is used that is more than 20% efficacious, that is, the vaccine restores the immune deficit in HD patients by 20% relative to healthy adults, then the adjuvanted strategy dominates the non-adjuvanted strategy during influenza seasons with a clinical attack rate ≥15.0%. Should a vaccine adjuvant be developed that completely overcomes immunosenescence in these individuals, then it becomes a dominant strategy when the influenza attack rate is ≥12.5%. The $2 adjuvant dominates when the vaccine efficacy is ≥60% and the clinical attack rate is ≥15%. When the clinical attack rate in a given year reaches 20%, the adjuvanted vaccine is the dominant strategy regardless of the efficacy. Should the influenza clinical attack rate reach 20%, the $5 adjuvant strategy is cost-effective given any adjuvant efficacy ≥20% and is the dominant strategy when the adjuvant efficacy is ≥40%.
Influenza Outcomes
Sensitivity analyses reveal that model outcomes are robust to the probability of clinic visit when sick with influenza. Our baseline scenario utilized a 40% probability of clinic visit26. When adjuvant efficacy is 100%, a $1 adjuvant remains economically dominant when ranging the probability of clinic visit from 20% to 80%, and the ICER of a $2 adjuvant ranges from $38,049/QALY to $49,283/QALY. When adjuvant efficacy is 80%, a $1 adjuvant ranges from being economically dominant when probability of clinic visit is 80% to having an ICER of $22,460/QALY when the probability of clinic visit is 20%. The ICER of a $2 adjuvant ranges from $52,868/QALY to $86,299/QALY. For the baseline influenza scenario, the expected value of perfect information given a $50,000 willingness to pay threshold was $1.18. This means an investment ≤$1.18 would be merited to collect perfect information on the uncertainties in the model.
The model is also relatively robust to changes in the probabilities of hospitalization and death. When adjuvant efficacy is 100%, the ICER for a $1 adjuvant varies from $23,334/QALY to $31,330/QALY when probability of hospitalization is half to a quarter of the baseline values remains economically dominant when ranging the probability of clinic visit from 20% to 80%, and the ICER of a $2 adjuvant ranges from $38,049/QALY to $49,283/QALY. When adjuvant efficacy is 80%, a $1 adjuvant ranges from being economically dominant when probability of clinic visit is 80% to having an ICER of $22,460/QALY when the probability of clinic visit is 20%. The ICER of a $2 adjuvant ranges from $52,868/QALY to $86,299/QALY.
In some cases, changing the probability of hospitalization does slightly change the threshold at which adjuvanted vaccine becomes cost-effective. For example, a 100% efficacious $2 adjuvant has an ICER of $38,357/QALY for the baseline hospitalization risk but ICERs of $80,100/QALY and $70,405/QALY when hospitalization risk is multiplied by 0.25 and 0.5, respectfully. Increasing the probability of hospitalization by two fold or more dropped the ICER to make the adjuvanted strategy cost-effective regardless of adjuvant cost. Multiplying the baseline hospitalization risk by ten (i.e., 12%) makes a ≤$2 adjuvant dominant for all possible efficacies and even a $5 adjuvant efficacy with ≥60% efficacy dominant.
Sensitivity analyses demonstrate that varying the influenza mortality has little effect on model outcomes. Dropping mortality by half or three-quarters does not change the thresholds at which the adjuvanted vaccine's ICER falls below $50,000/QALY. Even when the mortality is increased 10 fold, the cost-effectiveness threshold changes in only three scenarios: when the cost of the adjuvant is $1 and the adjuvant potency is 40% (from an ICER of $85,323/QALY to $32,326/QALY), and when the cost of the adjuvant is $2 and the adjuvant potencies are 60% and 80% (from ICERs of $99,801/QALY and $53,634/QALY to $40,158/QALY and $26,302/QALY, respectively).
Requirement for a Second Dose of Adjuvanted Vaccine
Requiring a second dose of vaccine to achieve full protection further attenuated the economic value of an adjuvanted influenza vaccine so that adjuvanted influenza vaccine was never a cost effective alternative over non-adjuvanted influenza vaccine (i.e., the ICER was well above $50,000/QALY) for baseline influenza risk scenarios. For example, a one dollar adjuvant and 75% patient adherence with receiving the second vaccine dose yielded ICER values ranging from $579,065 to $2,936,430 per QALY for adjuvant potencies of 25% to 100%, respectively.
Discussion
Our results indicate that an adjuvanted vaccine could be cost-effective in the HD population but that its economic value would be highly dependent on the adjuvant cost and efficacy. In order to be cost-effective, an adjuvant should be at least 60% efficacious in overcoming the gap between vaccine responses in HD patients and healthy adults. In all cases, the cost of the adjuvant (above and beyond the cost of the standard influenza vaccine) would have to be ≤$2 to be cost-effective in a standard influenza season. A more expensive or less potent adjuvant could be cost-effective in a year with a higher clinical attack rate (e.g., pandemic).
Adjuvanted influenza vaccines for the general older adult population are currently licensed in Europe and under late clinical development for the United States. A previously published computer model suggests that an adjuvanted vaccine would provide significant economic value in this population. A population of immunocompromised patients could be a likely next target with HD patients being a possibility. Patients undergoing chronic HD compromise a significant portion of the United States population (>1 per 1,000), and USRDS data trends suggest that this proportion will rise over the next decade10. These individuals are less capable of fighting off infection and are especially vulnerable to seasonal influenza. Patients on HD may experience a variety of immune deficiencies, including aberrant natural killer cell function, disruption of acquired T lymphocyte-dependent acquired immunity due to impaired antigen processing, preactivation and premature apoptosis of lymphocytes and monocytes, decreased B-lymphocyte counts, and altered cytokine profiles27-30.
Although there are currently no vaccine influenza adjuvants approved for use in the United States, successful studies looking at adjuvants in Hepatitis B vaccines for HD patients are a source of optimism that similar results could be seen with influenza vaccines in the future. Several studies cite granulocyte macrophage-colony stimulating factor (GM-CSF), for example, as being both safe and efficacious at providing seroprotective anti-Hepatitis B antibody titers in individuals with HD31-36. Studies involving the seasonal influenza vaccine have been somewhat less promising. MF-59, an oil-in-water emulsion adjuvant manufactured by Novartis, is one vaccine that is licensed for use in Europe and has been shown to be effective in the older adult population11-18. However, when tested in HD patients, the MF-59 adjuvant in one study failed to show a significant improvement in antibody response37. Another viable candidate is an AS03A oil-in-water emulsion produced by GlaxoSmithKline. The vaccine adjuvant is licensed for use in the European market and has been used as an H5N1 and H1N1 pre-pandemic/pandemic vaccine adjuvant17. Although the adjuvant has been shown to be safe and highly immunogenic in young children, adolescents and healthy adults,38-46 a report on its use in chronic HD patients has only just become available.46a Clinical trials are underway to examine other potential candidates. Thymosin alpha 1, a hormone produced by the thymus that has already been studied as an immune modulator for HIV patients, is among one of the current drugs being tested for efficacy in the HD population18.
Our study results are not exclusive to vaccine adjuvants but may apply to any method that could restore the immune response of adult HD patients to influenza vaccine. This includes systemic medications that can boost the overall immune system. There are also behavioral interventions such as dietary changes, exercise and stress reduction. Our study may also be applicable to populations that suffer immunosuppression similar to HD patients including patients with chronic obstructive pulmonary disease (COPD), diabetes mellitus, severe asthma, hepatic insufficiency, etc.
Assessing the value of a vaccine well before it reaches the market can help guide development and prepare the market to enhance its potential for success. Not doing so can lead to underutilization of a vaccine which can have wide-ranging negative public health effects47. Clinicians, scientists, vaccine developers and manufacturers, and policy makers could benefit from such an analysis.
A model is a simplification of real life and cannot represent all possible factors that may enter a decision. The HD population is extremely diverse and could have a wide range of possible outcomes. We limited our analysis to a single year and did not include certain potential longer-term benefits of influenza vaccination such as lowered heart disease risk that has been described in previous studies.48 Our model tried to represent the average HD patient. While sensitivity analyses explored the effects of some variation, they cannot capture the full possible extent of variability. Data inputs came from a variety of sources of varying quality. Influenza vaccine efficacy data came from humoral responses to the vaccine. Seroprotection values may not translate purely into clinical outcomes.
An adjuvanted influenza vaccine with adjuvant cost ≤$2 could be cost-effective in a standard influenza season depending on the efficacy of the adjuvant. Such a technology could fill an essential need as influenza is an important problem among HD patients. Results from our model could help guide vaccine development, as well as clinical utilization and reimbursement should adjuvanted influenza vaccines be licensed for this target population.
Acknowledgements
Support: This study was supported by the National Institute of General Medical Sciences Models of Infectious Agent Study (MIDAS) through grant 1U54GM088491-0109, the Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation, the National Library of Medicine through grant 5R01LM009132-02, and the Centers for Disease Control and Prevention (CDC) through grant 1P01HK000086-01.
Financial Disclosure: Dr Yee previously served as a consultant for GlaxoSmithKline and Novartis for other influenza vaccine products/indications. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001 Dec;120(6):1883–1887. doi: 10.1378/chest.120.6.1883. [DOI] [PubMed] [Google Scholar]
- 2.Naqvi SB, Collins AJ. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis. 2006 Jul;13(3):199–204. doi: 10.1053/j.ackd.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Gilbertson DT, Unruh M, McBean AM, Kausz AT, Snyder JJ, Collins AJ. Influenza vaccine delivery and effectiveness in end-stage renal disease. Kidney Int. 2003 Feb;63(2):738–743. doi: 10.1046/j.1523-1755.2003.00787.x. [DOI] [PubMed] [Google Scholar]
- 4.Vogtlander NP, Brown A, Valentijn RM, Rimmelzwaan GF, Osterhaus AD. Impaired response rates, but satisfying protection rates to influenza vaccination in dialysis patients. Vaccine. 2004 Jun 2;22(17-18):2199–2201. doi: 10.1016/j.vaccine.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Rautenberg P, Teifke I, Schlegelberger T, Ullmann U. Influenza subtype-specific IgA, IgM and IgG responses in patients on hemodialysis after influenza vaccination. Infection. 1988;16(6):323–328. doi: 10.1007/BF01644539. [DOI] [PubMed] [Google Scholar]
- 6.Cavdar C, Sayan M, Sifil A, et al. The comparison of antibody response to influenza vaccination in continuous ambulatory peritoneal dialysis, hemodialysis and renal transplantation patients. Scand J Urol Nephrol. 2003;37(1):71–76. doi: 10.1080/00365590310008749. [DOI] [PubMed] [Google Scholar]
- 7.Beyer WE, Versluis DJ, Kramer P, Diderich PP, Weimar W, Masurel N. Trivalent influenza vaccine in patients on haemodialysis: impaired seroresponse with differences for A-H3N2 and A-H1N1 vaccine components. Vaccine. 1987 Mar;5(1):43–48. doi: 10.1016/0264-410x(87)90008-9. [DOI] [PubMed] [Google Scholar]
- 8.Antonen JA, Hannula PM, Pyhala R, Saha HH, Ala-Houhala IO, Pasternack AI. Adequate seroresponse to influenza vaccination in dialysis patients. Nephron. 2000 Sep;86(1):56–61. doi: 10.1159/000045713. [DOI] [PubMed] [Google Scholar]
- 9.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009 Aug;9(8):493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Renal Data System . USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: 2009. [Google Scholar]
- 11.Baldo V, Menegon T, Bonello C, Floreani A, Trivello R. Comparison of three different influenza vaccines in institutionalised elderly. Vaccine. 2001 May 14;19(25-26):3472–3475. doi: 10.1016/s0264-410x(01)00060-3. [DOI] [PubMed] [Google Scholar]
- 12.Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology. 2003 May-Jun;49(3):177–184. doi: 10.1159/000069172. [DOI] [PubMed] [Google Scholar]
- 13.De Donato S, Granoff D, Minutello M, et al. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999 Aug 6;17(23-24):3094–3101. doi: 10.1016/s0264-410x(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Fang H, Li Y, Liu Y, Pellegrini M, Podda A. Safety and immunogenicity of an MF59-adjuvanted subunit influenza vaccine in elderly Chinese subjects. Immun Ageing. 2008;5:2. doi: 10.1186/1742-4933-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puig Barbera J, Gonzalez Vidal D. MF59-adjuvanted subunit influenza vaccine: an improved interpandemic influenza vaccine for vulnerable populations. Expert Rev Vaccines. 2007 Oct;6(5):659–665. doi: 10.1586/14760584.6.5.659. [DOI] [PubMed] [Google Scholar]
- 16.Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine. 2003 Mar 7;21(11-12):1268–1274. doi: 10.1016/s0264-410x(02)00401-2. [DOI] [PubMed] [Google Scholar]
- 17.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010 Jun;22(3):411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Trials.gov [Accessed June 7, 2009]; www.clinicaltrials.gov.
- 19.Lee BY, Ercius AK, Smith KJ. A predictive model of the economic effects of an influenza vaccine adjuvant for the older adult (age 65 and over) population. Vaccine. 2009 Apr 6;27(16):2251–2257. doi: 10.1016/j.vaccine.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepard DS. Cost-effectiveness in Health and Medicine. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. J Ment Health Policy Econ. 2. Vol. 2. Oxford University Press; New York: Jun 1, 1996. 1999. pp. 91–92. [Google Scholar]
- 21.Scharpe J, Peetermans WE, Vanwalleghem J, et al. Immunogenicity of a Standard Trivalent Influenza Vaccine in Patients on Long-term Hemodialysis: An Open-Label Trial. Am J Kidney Dis. 2009 Jul;54(1):77–85. doi: 10.1053/j.ajkd.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009 Jul 31;58(RR-8):1–52. [PubMed] [Google Scholar]
- 23.Diepersloot RJ, Bouter KP, Hoekstra JB. Influenza infection and diabetes mellitus. Case for annual vaccination. Diabetes Care. 1990 Aug;13(8):876–882. doi: 10.2337/diacare.13.8.876. [DOI] [PubMed] [Google Scholar]
- 24.Brydak LB, Machala M. Humoral immune response to influenza vaccination in patients from high risk groups. Drugs. 2000 Jul;60(1):35–53. doi: 10.2165/00003495-200060010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992 Feb 15;146(4):473–481. [PMC free article] [PubMed] [Google Scholar]
- 26.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 Jun 28;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Descamps-Latscha B. The immune system in end-stage renal disease. Curr Opin Nephrol Hypertens. 1993 Nov;2(6):883–891. doi: 10.1097/00041552-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995 Jan 15;154(2):882–892. [PubMed] [Google Scholar]
- 29.Pereira BJ, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994 Mar;45(3):890–896. doi: 10.1038/ki.1994.117. [DOI] [PubMed] [Google Scholar]
- 30.Zeltzer E, Bernheim J, Korzets Z, et al. Diminished chemokine and cytokine-induced adhesion of CD4+ T cells to extracellular matrix ligands in patients with end-stage renal failure. Isr Med Assoc J. 2000 Apr;2(4):282–286. [PubMed] [Google Scholar]
- 31.Anandh U, Bastani B, Ballal S. Granulocyte-macrophage colony-stimulating factor as an adjuvant to hepatitis B vaccination in maintenance hemodialysis patients. Am J Nephrol. 2000 Jan-Feb;20(1):53–56. doi: 10.1159/000013556. [DOI] [PubMed] [Google Scholar]
- 32.Fabrizi F, Ganeshan SV, Dixit V, Martin P. Meta-analysis: the adjuvant role of granulocyte macrophage-colony stimulating factor on immunological response to hepatitis B virus vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2006 Sep 1;24(5):789–796. doi: 10.1111/j.1365-2036.2006.03035.x. [DOI] [PubMed] [Google Scholar]
- 33.Jha R, Lakhtakia S, Jaleel MA, Narayan G, Hemlatha K. Granulocyte macrophage colony stimulating factor (GM-CSF) induced sero-protection in end stage renal failure patients to hepatitis B in vaccine non-responders. Ren Fail. 2001 Sep;23(5):629–636. doi: 10.1081/jdi-100107359. [DOI] [PubMed] [Google Scholar]
- 34.Singh NP, Mandal SK, Thakur A, et al. Efficacy of GM-CSF as an adjuvant to hepatitis B vaccination in patients with chronic renal failure--results of a prospective, randomized trial. Ren Fail. 2003 Mar;25(2):255–266. doi: 10.1081/jdi-120018726. [DOI] [PubMed] [Google Scholar]
- 35.Sudhagar K, Chandrasekar S, Rao MS, Ravichandran R. Effect of granulocyte macrophage colony stimulating factor on hepatitis-B vaccination in haemodialysis patients. J Assoc Physicians India. 1999 Jun;47(6):602–604. [PubMed] [Google Scholar]
- 36.Kapoor D, Aggarwal SR, Singh NP, Thakur V, Sarin SK. Granulocyte-macrophage colony-stimulating factor enhances the efficacy of hepatitis B virus vaccine in previously unvaccinated haemodialysis patients. J Viral Hepat. 1999 Sep;6(5):405–409. doi: 10.1046/j.1365-2893.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 37.Tanzi E, Amendola A, Pariani E, et al. Lack of effect of a booster dose of influenza vaccine in hemodialysis patients. J Med Virol. 2007 Aug;79(8):1176–1179. doi: 10.1002/jmv.20936. [DOI] [PubMed] [Google Scholar]
- 38.Carmona A, Omenaca F, Tejedor JC, et al. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6-35 months. Vaccine. 2010 Jun 29;28(36):5837–5844. doi: 10.1016/j.vaccine.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 39.Waddington CS, Walker WT, Oeser C, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. doi: 10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010 Jun 1;201(11):1644–1653. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 41.Diez-Domingo J, Garces-Sanchez M, Baldo JM, et al. Immunogenicity and Safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase ii, randomized, open, controlled study. Pediatr Infect Dis J. 2010 Jun;29(6):e35–46. doi: 10.1097/INF.0b013e3181daf921. [DOI] [PubMed] [Google Scholar]
- 42.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine. 2010 Feb 17;28(7):1740–1745. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz TF, Horacek T, Knuf M, et al. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009 Oct 23;27(45):6284–6290. doi: 10.1016/j.vaccine.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Leroux-Roels I, Roman F, Forgus S, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open nonrandomised extension of a double-blind randomised primary study. Vaccine. 2010 Jan 8;28(3):849–857. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Chu DW, Hwang SJ, Lim FS, et al. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009 Dec 9;27(52):7428–7435. doi: 10.1016/j.vaccine.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 46.Jones T. GSK's novel split-virus adjuvanted vaccines for the prevention of the H5N1 strain of avian influenza infection. Curr Opin Mol Ther. 2009 Jun;11(3):337–345. [PubMed] [Google Scholar]
- 46a.Dikow R, Eckerle I, Ksoll-Rudek D, et al. Immunogenicity and Efficacy in Hemodialysis Patients of a AS03A-Adjuvanted Vaccine for 2009 Pandemic Influenza A(H1N1): A Nonrandomized Trial. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2010.11.031. ••(•):•••-•••. [DOI] [PubMed] [Google Scholar]
- 47.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010 Apr 1;28(16):2806–2809. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder JJ, Collins AJ. Association of preventive health care with atherosclerotic heart disease and mortality in CKD. J Am Soc Nephrol. 2009 Jul;20(7):1614–1622. doi: 10.1681/ASN.2008090954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.PDR . Red Book. Thompson Healthcare Inc.; Montvale, NJ: 2009. [Google Scholar]
- 50.Centers for Medicare & Medicaid Services 2009 www.cms.hhs.gov. [PubMed]
- 51.National Compensation Survey: occupational wages in the United States, June 2006. U.S. Department of Labor; Washington, DC: 2007. [Google Scholar]
- 52.Characteristics of All Hospital Stays and Stays with a Principal Diagnosis of Influenza [Accessed 6/9/10];2005 www.hcup-us.ahrq.gov/reports.
- 53.Smith K, Roberts M. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002;113(September (4)):7. doi: 10.1016/s0002-9343(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 54.Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999 Apr 30;48(RR-4):1–28. [PubMed] [Google Scholar]
- 55.Windquist AG, Fukuda K, Bridges CB, Cox NJ. Neuraminidase inhibitors for treatment of influenza A and B infections. MMWR Recomm Rep. 1999 Dec 17;48(RR-14):1–9. [PubMed] [Google Scholar]
- 56.Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000 Mar 4;355(9206):827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- 57.Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med. 1997 Sep 25;337(13):874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 58.Jefferson TO, Demicheli V, Deeks JJ, Rivetti D. Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database Syst Rev. 2000;(2):CD001169. doi: 10.1002/14651858.CD001169. [DOI] [PubMed] [Google Scholar]
- 59.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000 Feb 23;283(8):1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 60.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000 Jun;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 62.Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Looijmans-Van den Akker I, Verheij TJ, Buskens E, Nichol KL, Rutten GE, Hak E. Clinical effectiveness of first and repeat influenza vaccination in adult and elderly diabetic patients. Diabetes Care. 2006 Aug;29(8):1771–1776. doi: 10.2337/dc05-2517. [DOI] [PubMed] [Google Scholar]
- 64.Clark TW, Pareek M, Hoschler K, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009 Dec 17;361(25):2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 65.Demicheli V, Rivetti D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2004;(3):CD001269. doi: 10.1002/14651858.CD001269.pub2. [DOI] [PubMed] [Google Scholar]