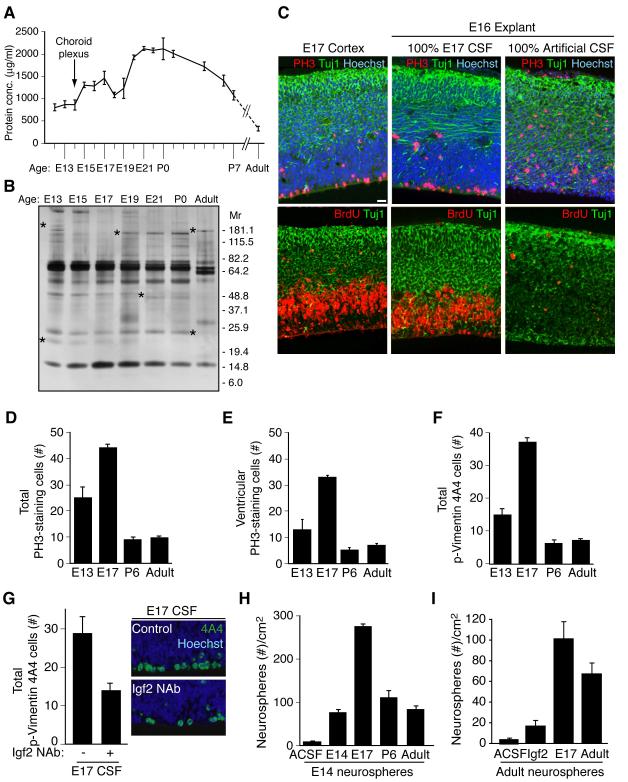
Figure 4. Embryonic CSF supports cortical explant viability and stimulates proliferation of neural progenitor cells.
(A) Total CSF protein concentration over rat development. (B) Silver stain of embryonic rat CSF revealed a dynamic fluid with numerous changes in protein composition over time. Asterisks indicate proteins with varying CSF expression during development. (C) E17 rat cortex and E16 explants grown for 24 hours in 100% embryonic E17 CSF or 100% artificial CSF respectively. Upper panels: anti-PH3 (red), and anti-Tuj1 (green), Hoechst (blue) immunostaining. Lower panels: anti-BrdU (red), and anti-Tuj1 (green) immunostaining. Explants cultured in 100% E17 CSF in vitro maintained tissue histology similar to embryo in vivo. Survival and proliferation of explants cultured with E17 CSF indicated by immunoreactivity for PH3 along the ventricular surface, BrdU incorporation in the ventricular zone, and Tuj1-positive-staining neurons in the developing cortical plate. (D) E16 explants cultured in 100% E13, E17, P6, or adult CSF for 24 hours were immunostained with anti-PH3 (red) and Hoechst (blue)(see Figure S2C). Quantification of total PH3-positive-staining cells per 400μm explant showed that proliferating cells increased in explants cultured with E17 CSF compared to E13, P6, or adult CSF. Immuno-positive cells are represented as mean ± SEM (E17 mean: 44.1 ± 1.43; E13 mean: 25 ± 4.2; P6 mean: 9.2 ± 0.8; adult mean: 9.6 ± 0.9, n = 4; Kruskal-Wallis; p<0.005). (E) Quantification of ventricular PH3-staining cells in explants (panel D). PH3-positive cells along the ventricle were significantly increased in explants cultured with E17 CSF compared to E13, P6, or adult CSF (E17 mean: 32.3 ± 0.79; E13 mean: 12.8 ± 3.9; P6 mean: 4.9 ± 1.0; adult mean: 6.9 ± 0.73; n = 4; Kruskal-Wallis; p<0.01). (F) E16 explants (panel D) immunostained with anti-Vimentin 4A4 (green)(see Figure S2C) were quantified. Vimentin 4A4-positive cells were significantly increased in explants cultured with E17 CSF compared to E13, P6, or adult CSF (E17 mean: 37.1 ± 1.4; E13 mean: 14.9 ± 1.9; P6 mean: 6.1 ± 1.05; adult mean: 7.3 ± 0.6; n = 4; Kruskal-Wallis; p<0.005). (G) Left panels: E16 explants cultured in control E17 CSF or E17 CSF with Igf2 neutralizing antibody (Igf2 NAb), immunostained with anti-Vimentin 4A4 and Hoechst (E17 control mean: 28.8 ± 4.3; E17 IGF2 NAb mean: 13.9 ± 2.0; n = 4; Mann-Whitney; p<0.05). Vimentin 4A4-positive cells decreased in explants cultured with E17 CSF plus Igf2 NAb compared to control. Right panels: Representative images of explants quantified in left panels. (H) Primary neurospheres derived from E14.5 cortex were grown in 20% E13/E14, E17, P6, or adult CSF for 10 days in vitro (DIV). E17 CSF generated the most spheres/cm2 (E17 mean: 274 ± 8.0; E13 mean: 77 ± 7.0; P6 mean: 110 ± 17.5; adult mean: 81 ± 8.8; n = 3; ANOVA; p<0.005). See also Figure S2. (I) Neurospheres derived from adult rat SVZ were cultured in artificial (A)CSF, Igf2 (20ng/ml), E17 CSF, or adult rat CSF for 10DIV. Igf2, E17 CSF, and adult CSF supported the growth and maintenance of adult neurospheres (ACSF: 4.76 ± 0.67; Igf2: 17.3 ± 3.2; E17 CSF: 101.7 ± 15.8; Adult CSF: 67.8 ± 12.6; Kruskal-Wallis: Igf2 vs. E17 CSF, p<0.05; E17 CSF vs. Adult CSF, N.S.; n=3). See also Figure S2.