Abstract
Ovarian hormones are associated with binge eating in women, however findings are limited by the lack of experimental control inherent in human studies. Animal research that manipulates ovarian hormone status and examines individual differences in extreme binge eating proneness are needed to model clinical phenotypes in humans and to confirm causal effects. The purpose of this study was to examine the effects of adult ovariectomy on overall binge eating risk and extreme binge eating phenotypes using the binge eating resistant (BER)/ binge eating prone (BEP) rat model. We predicted that palatable food consumption would significantly increase after ovariectomy in all rats because ovarian hormones generally suppress food intake. If differences in responsiveness to ovarian hormones underlie BER/BEP phenotypes, then differences in binge eating between BER and BEP rats would be eliminated or diminished after ovariectomy. Changes in palatable food (PF) intake were compared in BER and BEP rats before and after ovariectomy in two samples of adult females. Findings were highly similar in the two samples. PF intake increased significantly following ovariectomy in all rats. However, BEP rats consistently consumed larger amounts of PF than BER rats, both before and after ovariectomy. The consistency of findings across two samples of rats provides strong support for activational effects of ovarian hormones on binge eating. However, the immunity of extreme binge eating phenotypes to ovarian hormone ablation suggests that other, earlier mechanisms (e.g., organizational hormone effects or hormone-independent effects) determine the expression of binge eating phenotypes.
Keywords: binge eating, bulimia nervosa, ovariectomy, animal models, ovarian hormones
Eating disorders are significant mental health problems that affect over 5 million people in the United States (NIMH, 1994). Collectively, they are associated with the highest mortality rates of any psychiatric disorder (Harris and Barraclough, 1998) and tend to have chronic courses characterized by significant psychiatric and medical morbidity (Keel and Herzog, 2004). The decidedly bleak psychosocial outcomes associated with eating disorders (American Psychiatric Association, 2000; Keel and Herzog, 2004) further attest to their public health significance and the urgent need to understand their development.
The most prevalent forms of eating disorders are those characterized by binge eating (Keel et al., 2005; Wade et al., 2006), which is the consumption of an unusually large amount of food within a discrete period and a loss of control over eating during the episode (American Psychiatric Association, 2000). Binge eating is the cardinal symptom of bulimia nervosa (BN) and related eating disorder(s) not otherwise specified (e.g., binge eating disorder) (American Psychiatric Association, 2000). In addition to its significance for understanding bulimic syndromes, binge eating is an important target for research because it is a risk factor for weight gain and obesity (Stice et al., 1999; Stice et al., 2002).
Bulimic syndromes are much more common in females than males, with a sex ratio of approximately 4-10 females to every 1 male (American Psychiatric Association, 2000; Hudson et al., 2007). Differences in sex hormone concentrations may help explain the preponderance of eating disorders in women and account for patterns of initiation, remission, and relapse across the lifespan. In early life, puberty marks the beginning of peak risk for bulimic symptoms (American Psychiatric Association, 2000), and bulimic syndromes rarely occur in pre-pubertal individuals (Bulik, 2002). In adulthood, the behavioral symptoms of BN typically remit during pregnancy despite worsening cognitive symptoms (Crow et al., 2004) and then return to pre-pregnancy levels during the post-partum period (Crow et al., 2004; Lacey and Smith, 1987; Morgan et al., 1999; Willis and Rand, 1988). Finally, bulimic syndromes rarely begin after age 40 (American Psychiatric Association, 2000), being almost non-existent in older, post-menopausal women. These patterns of initiation, relapse, and remission suggest that ovarian hormones are prime candidates as mediators of neurobiological contributions to bulimic syndromes in women.
Similar to epidemiological patterns for eating disorders in humans, sex differences in feeding have been observed in animals. Male rats tend to eat more (Asarian and Geary, 2006; Wade, 1972) and experience smaller day-to-day fluctuations in feeding behaviors than females (Asarian and Geary, 2006; Wade, 1972). Gonadal hormones were initially hypothesized to account for these sexually dimorphic patterns, and research over the last 30 years has confirmed this hypothesis. Data across animal species are remarkably consistent in showing that decreases in estradiol, and increases in progesterone, cause increases in food intake in females (Asarian and Geary, 2006; Eckel, 2004; Varma et al., 1999; Wade, 1972, 1975). These effects have been observed in experimental manipulations of hormones as well as longitudinal studies examining fluctuations in food intake across the ovarian cycle (Asarian and Geary, 2006; Eckel, 2004; Varma et al., 1999; Wade, 1972, 1975).
Recent research has begun to investigate effects of ovarian hormones on binge eating risk in women. Studies examining predictive associations between changes in ovarian hormones and changes in binge eating across the menstrual cycle have shown that circulating estradiol concentrations are inversely, and progesterone concentrations are positively, associated with binge eating in women with BN as well as non-clinical samples of women (Edler et al., 2007; Klump et al., 2008). These associations are present even after controlling for menstrual cycle changes in negative affect and body weight, suggesting specific effects of ovarian hormones on binge eating risk (Edler et al., 2007; Klump et al., 2008).
Despite the consistency of these results, studies thus far are somewhat limited by the lack of experimental control – because ovarian hormone concentrations are difficult to manipulate in humans, conclusions are tempered by cautions that even longitudinal, prospective data in humans cannot definitively determine causal relationships. Experimental data are needed to confirm a causal role for ovarian hormones. Indeed, changes in binge eating following ovarian hormone ablation (via ovariectomy, OVX) would provide strong evidence for causal associations between these hormones and binge eating risk.
In the single animal study to examine this issue, Yu et al. (2008) found support for significant tonic effects of ovarian hormones on fat intake under binge eating conditions (e.g., intermittent access to high-fat food) in ovariectomized female rats. Specifically, fat intake was reduced by exogenous administration of a combined estradiol and progesterone treatment to OVX female rats given intermittent access to high-fat food (Yu et al., 2008). Effects were present in rats from low-restriction (i.e., 1 hour access to fat daily) as well as high restriction groups (i.e., 1-hour access to fat on Mondays, Wednesdays, and Fridays) (Yu et al., 2008). Overall, findings provide initial support for causal associations between ovarian hormones and binge-like behaviors in female rats.
Nonetheless, additional experimental research is needed to replicate these results and further elucidate causal associations. In particular, experimental research that models natural individual differences in binge eating risk as it appears in humans is needed to understand how and if ovarian hormones influence binge eating phenotypes. Animal research of binge eating has largely focused on group comparisons where between-group effects (i.e., rats with versus without experimental manipulation) are examined rather than between-rat individual differences in response to experimental manipulations (Asarian and Geary, 2006; Yu et al., 2008). A between-groups approach is useful for identifying initial effects, but it does not map well onto binge eating risk as it is expressed in humans, where individuals vary in their propensity to engage in binge eating (Bulik et al., 1998; Klump et al., 2010; Klump et al., 2000; Reichborn-Kjennerud et al., 2004), despite similar exposure to environmental “risks” (e.g., access to highly palatable food). Indeed, while binge eating exists on a continuum (ranging from low to high) in women, some women are binge prone while others are binge resistant, despite relative similarity in access to food and other environmental circumstances.
We directly addressed this issue by examining the effects of ovariectomy on binge eating in an individual differences model of binge eating risk. We used the binge eating resistant (BER)/binge eating prone (BEP) rat paradigm that models a continuum of binge eating (from low to high), while also identifying more extreme groups of binge eating resistant (BER) and binge eating prone (BEP) female rats in adulthood (Boggiano et al., 2007). BER rats consistently consume small amounts of intermittently presented PF across testing days, while BEP rats consistently consume high amounts of PF across testing days (Boggiano et al., 2007; Oswald et al., in press). Importantly, these individual differences in binge proneness are present in the absence of external manipulations that lead to binge eating in the majority of rats (e.g., food restriction). Thus, individual differences in the BER/BEP model reflect the same type of natural individual differences in binge eating risk present in women.
Several other aspects of the BER/BEP model make it ideal for examining binge eating, as it is expressed in humans. BEP rats binge eat on highly PF, but do not binge eat on standard rat chow (a less PF) (Boggiano et al., 2007; Oswald et al., in press). BEP rats also appear to experience a lack of control over their binge episodes, as they endure increasingly high levels of pain (via foot shock) in order to consume PF (Oswald et al., in press). In contrast, BER rats will not endure incremental foot shock in order to consume PF (Oswald et al., in press). Like women with BN, BEP rats tend to be of normal weight and do not differ significantly from BER rats in body weight or rates of diet-induced obesity (Boggiano et al., 2007; Oswald et al., in press). Recent research indicates that the BER/BEP phenotypes emerge during puberty (Klump et al., submitted), a pattern that is very similar to the development of binge eating in humans (American Psychiatric Association, 2000; Bulik, 2002; Garber et al., 1994). BEP rats also are more likely to binge eat in the presence of risk factors (Boggiano et al., 2007; Oswald et al., in press). For example, the effect of stress in BER/BEP rats closely resembles that in women for both the BER (i.e., they are unlikely to binge eat, even in the presence of stress) and BEP group (e.g., they are more likely to binge eat and increase binge eating in the presence of stress). Although data on sex differences in the BER/BEP model are currently lacking, on-going experiments in our lab have found a higher rate of BEP phenotypes in female versus male rats (Klump et al., in preparation).
Using the BER/BEP individual differences model, we examined changes in binge eating following OVX in adulthood in female rats. We predicted that OVX would increase overall palatable food intake in both BER and BEP rats. This hypothesis was based on previous studies of general food intake showing that the removal of estradiol via OVX increases food intake across a variety of species. We further expected that OVX would eliminate or reduce differences in PF intake between BER and BEP phenotypes, because if individual differences in binge eating proneness stem from individual differences in responses to ovarian hormones, then such differences will be observable only in the presence of hormones. This last hypothesis represents an initial step towards testing whether the expression of extreme binge eating phenotypes that are similar to those observed in humans (e.g., women with BN) are dependent upon the presence of circulating hormones in adulthood. In order to confirm that effects were robust, we examined all study hypotheses in two independent samples of rats followed longitudinally before and after OVX in adulthood.
Material and Methods
Animals
A total of 63 (n = 27 for Experiment1, n = 36 for Experiment 2) weanling female Sprague-Dawley rats were obtained from Harlan (Madison, Wisconsin). These rats were part of a larger experiment examining changes in binge eating patterns from pre-puberty into adulthood (Klump et al., submitted). The animals arrived in our laboratories on postnatal day 19 (i.e., P19), although the current paper is focused on the period of adulthood (P60-P86) only.
Animals were singly housed in clear Plexiglas cages (45 × 23 × 21 cm) and given continuous ad lib access to water and chow (Rodent diet 8640; Harlan Teklad Global Diets, Madison, WI) provided in a wire cage lid. Animals were maintained on a 12/12 hr light-dark cycle (lights on at 2400h; off at 1200h) and the temperature was maintained at 21 ± 2°C. All animals were treated in accordance with the NIH Guide for the Care and use of Laboratory Animals, and all protocols were approved by the Michigan State Institutional Animal Care and Use Committee.
Experimental Design
Experiment 1
Feeding tests followed slight modifications of BER/BEP protocols (Boggiano et al., 2007; Oswald et al., in press). Prior to lights out on testing days, 24 hour chow and spillage were measured, discarded, and replaced with: 1) approximately 50-80 grams of whole chow pellets (whole chow pellets were used to make it easy to locate spillage); and 2) approximately 15-20 grams of Betty Crocker Creamy Vanilla Frosting (General Mills Inc., Minneapolis, MN) inside a small Petri dish fitted with a wire hook and hung on the inside of the cage. Petri dishes of PF and chow were then weighed at the 1, 2, 4, and 24-hour time points using an electronic balance and rounded to the nearest tenth of a gram. Before each weighing, the bedding was searched for chow spillage, and all chow found in the bedding was added to the rest of the chow before weighing. The petri dish of PF was removed after the 24 hour time point until the next feeding test. Body weight was measured every day prior to lights out.
Unlike the original BER/BEP model, feeding tests were given 3x/week (Monday, Wednesday, Friday) in the current study rather than 1-2 times per week (Boggiano et al., 2007; Oswald et al., in press). This modification was necessary to accommodate other specific aims of the experiments, i.e., to examine changes in binge eating proneness during puberty (Klump et al., submitted). These more frequent feeding tests were required in order to capture changes in PF intake over the relatively brief pubertal period. Importantly, however, these changes did not seem to unduly influence our results, as our proportion of BER/BEP rats and the pattern of chow intake and body weight is highly similar to that observed previously (see Results below and Boggiano et al. (2007) and Oswald et al. (in press) for previous work).
Nine feeding tests were given in adulthood. After the first 5 adult feeding tests, ovariectomies were performed on days P70 and P71 (~15 animals per day). Animals were anesthetized with isofluorane (Abbott Laboratories, North Chicago, IL) between 0900h and 1100h at which time ovaries were surgically removed according to standard procedures. Feeding tests were then suspended for 7 days while rats recovered from the surgery. Feeding tests were re-started on P79 and continued until day 86 for a total of 4 feeding tests during the post-OVX stage.
Experiment 2
Experiment 2 was identical to Experiment 1 with two exceptions. First, we conducted SHAM surgeries instead of OVX in 6 rats that were randomly selected from the full sample. These SHAM surgery rats enabled us to examine whether the effects of OVX were due to hormone removal rather than the simple passage of time. Although extant previous research shows that increases in food intake are due to the effects of ovariectomy rather than time (see Wade, 1976 #816), we wanted to confirm these OVX effects within our BER/BEP model. Sham surgeries included isofluorane anesthesia, incision through flank skin and muscle, suturing of the muscle, closing the skin incision with wound clips, and post surgical analgesia.
Second, we continued feeding tests (a total of 3 tests) during the surgery recovery phase. The purpose of this modification was to ensure that findings from Experiment 1 were not due to rebound hyperphagia effects following a week-long withdrawal of PF after the surgeries. Notably, although we continued feeding tests during the recovery period, these tests were not included in analyses, as the behavior of the rats would be expected to be confounded with the potential effects of surgery and post-operative analgesics on appetite.
Statistical Analyses
Establishing BER/BEP Groups
We followed the Boggiano et al. (2007) method for identifying BER and BEP rats by examining tertiles of 4-hour PF intake across the five feeding tests that took place during adulthood. Our focus on the 4-hour intakes comes from previous research with the BER/BEP model (Boggiano et al., 2007; Oswald et al., in press) and other rat models of binge eating (Boggiano et al., 2005; Hagan et al., 2003; Hagan et al., 2002) confirming that measurable binge eating can be consistently observed and measured during this time interval.
After establishing tertiles for PF intake on each individual feeding test day, we identified BER rats as those that ate in the lowest tertile of PF intake on at least three out of the five feeding test days (i.e., 60% of the feeding tests), but never ate in the highest tertile for any feeding test. By contrast, BEP rats were those that ate in the highest tertile of PF intake on 3 out of 5 testing days, but never ate in the lowest tertile during any feeding test. Notably, the proportion of BER or BEP rats in a population could range from 0-100% (i.e., there is no constraint on the number of BER/BEP rats identified) since the BER/BEP definition is based on the frequency and consistency of PF intake across testing days rather than PF tertiles for any given day. This focus on binge eating frequency and consistency closely follows methods for defining binge eating status in eating disorders (e.g., binge eating disorder) where women who binge eat at least 2x/week for three consecutive months (American Psychiatric Association, 2000) are considered to be binge eaters or “binge prone”, while those who rarely binge eat are considered non-binge eaters or “binge resistant”. Much like the BER/BEP model, the cut-offs for determining these binge eating groups were based on statistical comparisons of women at the high versus low end of the binge eating distribution (American Psychiatric Association, 1997).
Examining the Effects of OVX
Our primary analyses used mixed linear models (MLM) implemented in the Statistical Package for the Social Sciences (SPSS) Version 17.0 to compare changes in PF intake, chow intake, and body weight for BER versus BEP rats before and after OVX surgery. We used an autoregressive (lag 1) error structure to model the residual covariance from one feeding trial to the next. The upper-level unit for the MLM was the rat, and the lower-level unit was feeding test. BER/BEP status was an upper-level predictor, and hormonal condition (i.e., before versus after OVX) was a lower-level predictor. When the outcome was PF intake, we expected to find a significant main effect of OVX, such that levels of PF intake would increase significantly following OVX in both groups. We did not expect to find a significant main effect of BER/BEP status, because although PF intake would vary before OVX (since BER/BEP status was defined based on pre-OVX PF levels), we expected the removal of ovarian hormones to eliminate or decrease group differences in PF intake. Thus, we expected to find a significant OVX x BER/BEP group interaction, where group differences in PF intake would only be present before (but not after) OVX.
We conducted these same MLM analyses with chow intake and body weight as dependent variables. Given past research, we expected significant main effects of OVX for both variables, as food intake and body weight should increase with OVX. However, we did not expect significant main effects of BER/BEP group, or significant BER/BEP group x OVX interactions, since past research strongly suggests that BER and BEP rats do not differ in chow intake or body weight. We examined these hypotheses using chow intake and body weight on feeding test days only. However, patterns of chow intake and body weight were similar across non-feeding and feeding test days, with the exception that BER and BEP rats appeared to consume more chow on non-feeding test days (data not shown). This is likely due to the consumption of PF in addition to chow on feeding test days.
Examining the Effects of OVX, controlling for the Effects of Time
Finally, we examined the effects of time in all MLM models in a set of secondary analyses. In Experiment 1, where we didn't have a SHAM surgery control group, we conducted all of the MLM analyses a second time and added rat age (in days) as a lower-level predictor. These analyses examined whether OVX predicted PF intake, chow consumption, and body weight, over and above the effects of time. In Experiment 2, we added in the SHAM surgery control group and included “surgery type” (OVX versus SHAM) and pre-post surgery variables in place of the age variable in the models. These substitutions provided the strongest test of OVX, as we could then disambiguate the passage of time from the direct effects of OVX.
Results
Experiment 1
Identifying BER/BEP Rats
We identified 8 BER (8/27; 30%) and 9 BEP (9/27; 33%) rats during the baseline adult period. This proportion is on par with rates observed in previous work (Boggiano et al., 2007; Oswald et al., in press) and suggests that roughly 1/3 of rats consistently consume high levels of PF across testing days (BEP rats), 1/3 consistently consume low levels of PF (BER rats), and the remaining 1/3 were inconsistent in their PF intake and/or ate only moderate amounts of PF.
Examining the Effects of OVX
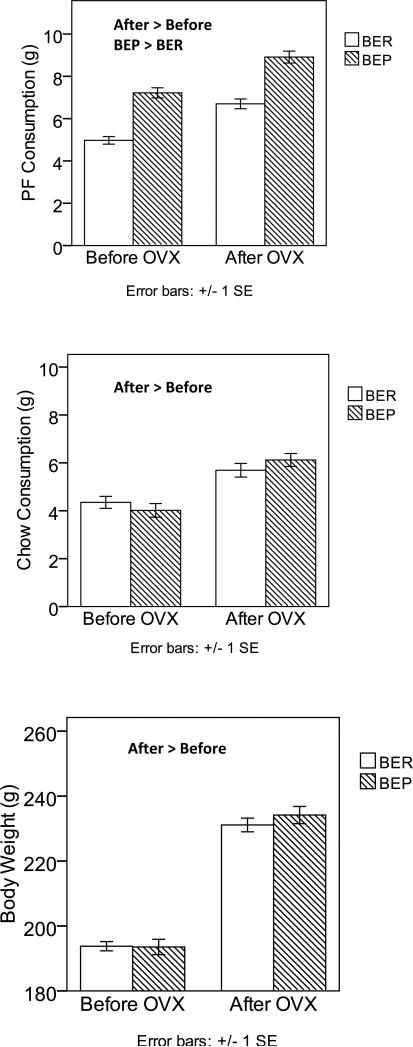
Table 1 includes means and standard deviations for BER/BEP rats before and after OVX, as well as F-tests from the MLM for the main effects of pre/post OVX and BER/BEP status, and the interaction between these two variables. As predicted, main effects for OVX status occurred for each of the three outcomes, indicating increased PF consumption, chow consumption and body weight after OVX surgery in both BER and BEP rats (see Table 1 and Figure 1). These findings suggest the presence of activational effects of ovarian hormone removal on all three variables.
Table 1.
Experiment 1 Means, Standard Deviations, and F-tests comparing BER Rats (N = 8 rats) to BEP (N = 9 rats) Rats before and after Ovariectomy.
| Adult Baseline | OVX | OVX Main Effect | BER/BEP Main Effect | OVX by BER/BEP Interaction | ||
|---|---|---|---|---|---|---|
| Palatable Food | F(1,73) = 56.02 p < .001 | F(1,48) = 74.60 p < .001 | F(1,73) = .00 p = .957 | |||
| BER | M (SD) | 4.85 (1.24) | 6.70 (1.30) | |||
| BEP | M (SD) | 7.01 (1.50) | 8.91 (1.69) | |||
| Chow | F(1,72) = 20.57 p < .001 | F(1,36) = .05 p = .820 | F(1 ,79) =.56 p = .456 | |||
| BER | M (SD) | 4.39 (1.79) | 5.69 (1.61) | |||
| BEP | M (SD) | 4.18 (2.02) | 6.12 (1.60) | |||
| Body Weight | F(1,131) = 112.38 p < .001 | F(1,11) = .12 p = .738 | F(1,131) = .15 p = .696 | |||
| BER | M (SD) | 194.19 (10.72) | 231.11 (11.95) | |||
| BEP | M (SD) | 196.24 (15.16) | 234.15 (15.89) | |||
Note. BER = binge eating resistant; BEP = binge eating prone. PF and chow intake were measured at the 4-hour time point, while body weight was measured at 24 hours. PF intake, chow intake, and body weight were all measured in grams.
Figure 1. Changes in PF Intake, Chow Intake, and Body Weight after Ovariectomy in BER and BEP Rats from Experiment 1.
Changes in PF consumption are shown in the top panel, changes in chow consumption are shown in the middle panel, and changes in body weight are shown in the bottom panel. Values are means with ± 1 standard errors. PF and chow intake were measured at the 4-hour time point, while body weight was measured at 24 hours. Groups that are significantly different from each other at p < .05 are indicated within the figure.
Interestingly, and counter to our hypothesis, there was a significant main effect of BER/BEP group status for PF consumption (but not chow or body weight – see Table 1), such that BEP rats continued to eat significantly more PF on average (M = 8.91, SD = 1.69) than did BER rats (M=6.70, SD = 1.30) after OVX. Figure 1 shows clear evidence of this main effect, as the BEP rats’ PF consumption was consistently about 2 to 2.5 grams higher than BER rats’ PF consumption, both before and after OVX. Moreover, the lack of a significant OVX x BER/BEP group interaction (see Table 1) indicates that the PF responses of BER and BEP rats to OVX were similar. These results imply that despite possible activational effects of ovarian hormone removal on PF intake in both BER and BEP groups, hormone ablation did not affect the underlying binge resistant or binge prone phenotypes. Rats maintained their two-group status even in the face of changing hormonal milieus and increasing overall levels of PF intake in both groups.
Examining the Effects of OVX, controlling for the Effects of Time
In secondary analyses, we tested whether the activational effects of OVX are present above and beyond the effects of time by including each rat's age (in days) as an additional lower-level predictor. Results for BER/BEP group and BER/BEP group x OVX interactions remained unchanged for all models, i.e., BEP rats continued to consume more PF than BER rats (F(1,50) = 81.56; p < .001) across all feeding tests but were not significantly different from BER rats in chow intake [F(1,36) = 0.05; p = .82] or body weight [F(1,15) = 0.46; p = .51].
However, results for the OVX main effects became largely non-significant (with the exception of body weight, where main effects of time [F(1,147) = 404.28; p < .001] and OVX [F(1,140) = 36.35; p < .001] were both significant). For PF intake, only the time main effect was significant [F(1,139) = 8.84; p = .003], and when time was included in the model, the main effect of OVX was no longer significant [F(1,143) = 0.05 p = .83]. For chow intake, neither the main effects of time [F(1,142) = 0.01; p = .91] nor OVX [F(1,144) = 2.46; p = .12] were significant. Collectively, these results demonstrate that we cannot definitively attribute the observed OVX effects to the surgery, as OVX and time are confounded. It is therefore difficult to tease apart their independent effects when they are included in the same model. Fortunately, the inclusion of SHAM rats in Experiment 2 allowed us to directly examine these possibilities by disambiguating time from the OVX surgery.
Experiment 2
Identifying BER and BEP Rats
In Experiment 2, we again identified a sizable number of BER (7/36; 19%) and BEP (11/36; 30%) rats in adulthood. Because one of the BER and two of the BEP rats fell in our SHAM control group, sample sizes for MLM analyses examining the effects of BER/BEP status and OVX were slightly reduced (BER N = 6; BEP N = 9).
Examining the Effects of OVX
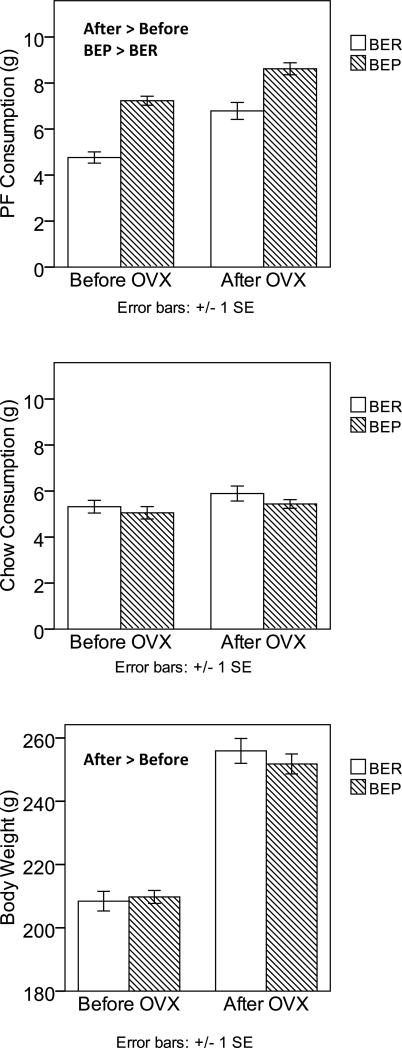
Table 2 includes means and standard deviations for BER/BEP rats before and after OVX, as well as F-tests from the MLM for the main effects of OVX and BER/BEP status, and their interaction. As in Experiment 1, there was evidence of a significant main effect for pre/post OVX surgery for PF consumption. Consumption of PF post-OVX surgery increased significantly relative to baseline (see Figure 2), suggesting a role for activational effects of hormone removal on PF intake. Similar findings were obtained for body weight (see Table 1). By contrast, chow consumption did not change with OVX in Experiment 2. Reasons for the lack of increase are unclear, although rats in Experiment 2 exhibited higher pre-OVX chow intake than those in Experiment 1. Indeed, while post-OVX chow consumption was similar in rats from Experiments 1 and 2 [Experiment 1 M = 5.91, SD = 1.61, Experiment 2 M = 5.62, SD = 1.34, F(1,45) = .57, p = .455], rats from Experiment 2 ate significantly more chow pre-OVX than rats in Experiment 1 [Experiment 1 M = 4.18, SD = 1.89, Experiment 2 M = 5.16, SD = 1.69, F(1,47) = 6.50, p = .014].
Table 2.
Experiment 2 Means, Standard Deviations, and F-tests comparing BER Rats (N = 6 rats) to BEP Rats (N = 9 rats) before and after Ovariectomy
| Adult Baseline | OVX | OVX Main Effect | BER/BEP Main Effect | OVX by BER/BEP Interaction | ||
|---|---|---|---|---|---|---|
| Palatable Food | F(1,87) = 18.99 p < .001 | F(1,25) =31.24 p < .001 | F(1,87) = .25 p = .619 | |||
| BER | M (SD) | 4.76 (1.33) | 6.79 (1.80) | |||
| BEP | M (SD) | 7.25 (1.26) | 8.34 (1.57) | |||
| Chow | F(1,92) = 1.88 p = .173 | F(1,25) = .32 p = .577 | F(1,92) = .06 p = .805 | |||
| BER | M (SD) | 5.32 (1.52) | 5.89 (1.60) | |||
| BEP | M (SD) | 5.05 (1.81) | 5.44 (1.14) | |||
| Body Weight | F(1,117) = 135.12 p < .001 | F(1,10) = .01 p = .936 | F(1,117) = .39 p = .535 | |||
| BER | M (SD) | 208.42 (16.94) | 255.92 (19.24) | |||
| BEP | M (SD) | 209.76 (13.78) | 251.80 (18.89) | |||
Note. BER = binge eating resistant; BEP = binge eating prone. PF and chow intake were measured at the 4-hour time point, while body weight was measured at 24 hours. PF intake, chow intake, and body weight were all measured in grams. Results exclude the six sham surgery rats.
Figure 2. Changes in PF Intake, Chow Intake, and Body Weight after Ovariectomy in BER and BEP Rats from Experiment 2.
Changes in PF consumption are shown in the top panel, changes in chow consumption are shown in the middle panel, and changes in body weight are shown in the bottom panel. Values are means with ± 1 standard errors. PF and chow intake were measured at the 4-hour time point, while body weight was measured at 24 hours. Groups that are significantly different from each other at p < .05 are indicated within the figure.
With regard to BER/BEP status, there continued to be a significant main effect of BER and BEP group for PF intake, such that BEP rats ate significantly more PF on average (M = 7.85, SD = 1.58) than did BER rats (M = 5.66, SD = 1.85), and this difference was similar in size both before and after OVX (see Figure 2). The OVX by BER/BEP group interaction was again non-significant, indicating that BER and BEP rats did not differ in their PF intake in response to OVX. Instead, they tended to maintain their two-group status despite hormone ablation.
Examining the Effects of OVX, controlling for the Effects of Time
In secondary analyses for Experiment 2, we disambiguated the effects of time from OVX by adding the SHAM rats into analyses. Because only half (3 rats) of the SHAM rats were categorized as BER (N = 1 rat) or BEP (N = 2 rats), we conducted this analysis with all of the rats from Experiment 2 (N = 36 rats; 30 received OVX, 6 received SHAM surgery), without the BER/BEP group variable in the model. This approach allowed us to include all 6 SHAM rats in analyses and was supported by our prior results showing that BER and BEP rats do not differ in their response to OVX for any of the dependent variables (e.g., all rats increased their PF intake after OVX; see above).
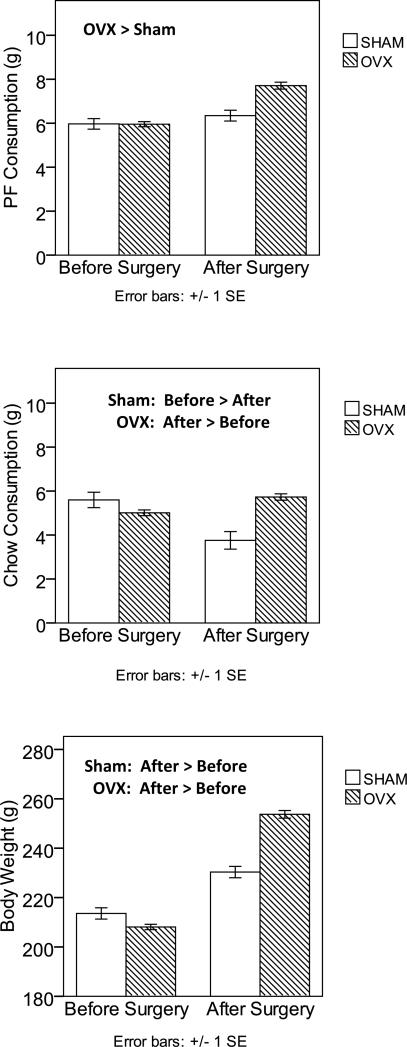
Results from these analyses are presented in Table 3 and Figure 3. Inspection of the means suggests that OVX surgery, rather than the passage of time, accounts for the significant OVX effects observed previously. For all three dependent variables, larger increases in PF intake, chow intake, and body weight were observed in the OVX group than in the SHAM group. These impressions were confirmed by the surgery type (OVX versus SHAM) x pre-post surgery interactions. This interaction failed to reach statistical significance for PF (p = .14), although effect sizes indicated that this is likely due to small sample sizes. Indeed, differences between the baseline and post-surgery means for the SHAM group were small effect (d = .30, F(1,46) = 2.23, p = .142), whereas these differences exhibited very large effect sizes (d = 1.12, F(1,220) = 37.07, p < .001) in the OVX group.
Table 3.
Experiment 2 Means, Standard Deviations, and F-tests comparing OVX Rats (N = 30 rats) to SHAM Rats (N = 6 rats) before and after Surgery.
| Before Surgery | After Surgery | Surgery Type Main Effect | Pre-Post Surgery Main Effect | Surgery Type × Pre-Post Interaction | ||
|---|---|---|---|---|---|---|
| Palatable Food | F(1,266) = 13.02 p < .001 | F(1,53) = 3.48 p = .068 | F(1,266) = 2.15 p = .144 | |||
| SHAM | M (SD) | 5.97 (1.31) | 6.35 (1.21) | |||
| OVX | M (SD) | 5.96 (1.41) | 7.71 (1.71) | |||
| Chow | F(1,198) = 3.30 p = .071 | F(1,77) = 3.20 p = .078 | F(1,198) =17.08 p < .001 | |||
| SHAM | M (SD) | 5.60 (1.92) | 3.76 (1.91) | |||
| OVX | M (SD) | 5.01 (1.60) | 5.73 (1.58) | |||
| Body Weight | F(1,282) = 114.09 p < .001 | F(1,27) = 1.89 p = .18 | F(1,282) = 27.18 p < .001 | |||
| SHAM | M (SD) | 213.57 (12.54) | 230.33 (11.31) | |||
| OVX | M (SD) | 208.09 (13.22) | 253.71 (16.94) | |||
Note. OVX rats = rats receiving ovariectomies; SHAM rats = rats receiving SHAM surgeries. PF and chow intake were measured at the 4-hour time point, while body weight was measured at 24 hours. PF intake, chow intake, and body weight were all measured in grams.
Figure 3. Changes in PF Intake, Chow Intake, and Body Weight in SHAM versus OVX Rats from Experiment 2.
Changes in PF consumption are shown in the top panel, changes in chow consumption are shown in the middle panel, and changes in body weight are shown in the bottom panel. Values are means with ± 1 standard errors. PF and chow intake were measured at the 4-hour time point, while body weight was measured at 24 hours. Groups that are significantly different from each other at p < .05 are indicated within the figure.
For both chow intake and body weight, the surgery type (OVX versus SHAM) by pre-post surgery interaction was significant, suggesting that rats in the OVX group increased their chow consumption (F(1,170) = 8.47, p = .004) and body weight (F(1,236) = 347.63, p < .00001) significantly more after surgery than SHAM rats (chow intake: F(1,28) = 8.54, p = .007; body weight: F(1,47) = 16.98, p < .0001) (see Figure 3). Notably, chow intake decreased in the SHAM group, which was an unexpected finding. Reasons for this decrease are unclear, although it seems likely that SHAM rats’ compensated for their modestly increased PF intake (see above) by lowering their chow intake, particularly given that the body weights of SHAM rats increased after surgery (see above).
Discussion
The present work capitalized on the existence of an individual differences model of binge eating to examine how levels of binge eating as well as extreme binge eating phenotypes respond to ovariectomy in adulthood. We found evidence for activational effects of ovarian hormones on consumption of intermittently presented PF in all rats. In contrast, extreme binge eating phenotypes appeared to persist after ovarian hormone removal, indicating that the BER and BEP phenotypes are not the consequence of individual differences in responsiveness to the activating effects of ovarian hormones on PF consumption.
We first tested the hypothesis that ovarian hormone ablation would increase consumption of PF in two independent samples of rats. This hypothesis was supported, in that PF intake significantly increased following OVX in both BER and BEP rats. Results for PF were consistent across the two independent cohorts of rats, indicating that these findings are replicable and represent robust changes in PF intake following OVX in all female rats. Perhaps more importantly, examination of SHAM rats in Experiment 2 confirmed that these effects were due to the OVX surgery rather than just the simple passage of time.
Ovariectomy had similar effects on body weight, with increases in body weight that followed OVX and were not due to time effects. Findings for chow intake were more variable. Increases in chow intake were observed after OVX in rats from Experiment 1, but rats in Experiment 2 failed to show expected increases. Reasons for this are unclear, given extant data showing increases in general food intake following OVX in female rats (Asarian and Geary, 2006; Varma et al., 1999; Wade, 1975). Higher chow intakes prior to OVX in Experiment 2 rats may have contributed to the lack of increase (see Results). Alternatively, differences across experiments could be due to differences in the degree of PF exposure. Rats from Experiment 2 experienced more feeding tests than rats in Experiment 1 as a result of continued feeding tests during the OVX recovery period (3 extra tests; see Methods) and extra feeding tests during development (i.e., approximately 5 extra tests) (Klump et al., submitted). How or why these additional feeding tests would alter the response of chow intake, but not PF intake, to OVX in rats in Experiment 2 is unclear. Additional research is needed to investigate this issue, particularly given previous studies showing that early exposure to sweet foods only increases sweet food consumption (not chow consumption) later in life (Silveira et al., 2008).
With regard to our findings for PF, data over the last 40 years indicate that the removal of estradiol, rather than the removal of progesterone, likely accounts for the activational effects of ovarian hormones on PF intake in both BER and BEP rats. Estradiol has anorexic effects on food intake (Asarian and Geary, 2006; Eckel, 2004; Varma et al., 1999; Wade, 1972, 1975), such that the removal of estradiol causes significant increases in food consumption in a variety of species. By contrast, in many animals, progesterone has small direct effects on food intake, but instead acts indirectly by antagonizing the effects of estrogen (Asarian and Geary, 2006; Blaustein and Wade, 1976; Gray and Greenwood, 1982; Marrone et al., 1975).
A note of caution is warranted, however. We did not study PF consumption in OVX rats after replacement with estradiol or progesterone, so we are unable to definitively conclude that it was the lack of estradiol that caused increased PF consumption following OVX. Likewise, previous animal studies of ovarian hormone effects on binge eating also have not separated the effects of estradiol from progesterone (Yu et al., 2008). These caveats are important given emerging data suggesting that progesterone may have more direct effects on binge eating in humans than previously believed. Studies examining binge eating patterns across the menstrual cycle find that progesterone is significantly and positively associated with changes in food intake and binge eating in women, even after controlling for the effects of negative affect and estradiol (Edler et al., 2007; Klump et al., 2008). These results indicate a potential lack of comparability across species with regard to progesterone's effects. Notably, however, the experimental designs used to examine progesterone's direct effects in women are more limited than those used in animals as 1) there is no menstrual cycle phase in women where progesterone is present but estrogen is not; and therefore 2) progesterone's direct effects are examined statistically (by regressing out estradiol levels prior to analyses) rather than experimentally (i.e., through OVX and exogenous progesterone treatment).
Nonetheless, understanding the possible presence and nature of cross-species differences in progesterone's effects will be an important area for future translational research. For example, women and non-human female primates experience a prolonged luteal phase that is not present in female rats. This lack of sustained periods of elevated progesterone in rats may weaken progesterone-food intake associations at the phenotypic as well as neural level. Experimental studies of non-human primates could help address this possibility, although relatively few studies have been conducted. Initial data suggest that food intake is higher during the luteal than the follicular or ovulatory phase of the menstrual cycle in female monkeys (Bielert and Busse, 1983; Czaja, 1975, 1978; Kemnitz et al., 1984; Kemnitz et al., 1989; Rosenblatt et al., 1980). However, progesterone treatment alone has only inconsistently affected food intake in ovariectomized monkeys (Czaja, 1978; Roth et al., 2005). Clearly, more research is needed to clarify potential species-specific effects across a range of models utilizing human, non-human primate, and rodent data collections.
Our second hypothesis examined whether OVX significantly alters the BER/BEP phenotype. We predicted that differences in PF intake between BER and BEP rats would be eliminated or reduced following OVX if extreme binge eating phenotypes were a function of individual differences in responsiveness to activational effects of ovarian hormones on the propensity to binge eat. This hypothesis was not supported. Rats in both experiments increased their PF intake after OVX, but they maintained their 2-group status, i.e., BEP rats continued to eat more PF than BER rats, even after OVX.1 These group differences were not due to differences in body weight or chow intake, as BER and BEP rats did not vary on these variables in either experiment. Thus, despite activational effects of OVX on overall consumption of PF in all rats, the more extreme phenotypes of binge eating resistance and binge eating proneness were not affected by hormone ablation in adulthood. On the contrary, there appeared to be permanent “marks” on the animals’ systems that made some rats consistently binge eat more, and some rats consistently binge eat less, regardless of the adult hormonal milieu. Understanding the nature of these “marks” will be another important area for future research, particularly given that BEP rats resemble women with BN in several binge eating characteristics (Boggiano et al., 2007; Klump et al., submitted; Oswald et al., in press) Although these permanent patterns of binge eating resistance/proneness may arise from non-hormonal mechanisms, we propose that they are due to the organizational (rather than activational) effects of ovarian hormones during earlier stages of development.
In its original conception, the organizational-activational framework posited that organizational effects of gonadal steroids occur during the prenatal/early postnatal period of development. However, it is now recognized that in addition to the perinatal period of hormone-dependent organization of neural circuits and behavior, adolescence is another period of development during which pubertal hormones organize the nervous system (Schulz et al., 2009; Sisk and Zehr, 2005). For example, food guarding is a sexually dimorphic behavior in rats, with adult males and females displaying different postural strategies for defending their food source (Field et al., 2004). Prepubertal ovariectomy significantly alters the defense strategy to be more male-like, whereas adult ovariectomy has no effect. Thus, these data suggest that ovarian hormones during the pubertal period actively feminize postural strategies for food defense. Another report demonstrates that pubertal estradiol feminizes ingestive responses to metabolic signals in rats (Swithers et al., 2008). Treatment with mercaptoacetate, a drug that interferes with fatty acid oxidation, causes an increase in food intake in adult male, but not female, rats. While OVX in adulthood has no effect on this sex difference, pre-pubertally OVX females show a male-like response to mercaptoacetate (i.e., increased food intake). This effect of prepubertal OVX can be prevented by treatment with estradiol during the time of puberty. Thus, the female adolescent brain is sensitive to organizational actions of ovarian hormones.
Our findings broadly fit with this pattern of results. In a previous study, we reported that the BER and BEP phenotypes emerge with puberty, such that no differences in PF intake are present in pre-puberty, but significant differences emerge during, and persist after, puberty (Klump et al., submitted) . In the current report, we find that OVX in adulthood has no effect on these BER/BEP phenotypes. Thus, as least two conditions (e.g., the emergence of the phenotype during puberty, and the permanence of the phenotype in the face of hormone ablation in adulthood) are present for an organizational effect. Importantly, one previous study also suggested the presence of organizational effects for binge eating. Zehr et al. (2007) found that early pubertal timing was associated with increased binge eating in adulthood. These findings imply organizational mechanisms, as the early exposure to ovarian hormones appeared to leave permanent “marks” on women's binge eating risk that persisted beyond the initial pubertal hormone exposure (Zehr et al., 2007).
In future research, it will be important to directly test our hypotheses regarding organizational effects of ovarian hormones during puberty on binge eating risk. An ideal test of this hypothesis would be to compare the emergence of the BER/BEP phenotype in intact rats versus OVX rats with and without exogenous hormone exposure during puberty and adulthood. The emergence of BER/BEP phenotypes in intact rats and only those OVX rats experiencing pubertal hormone replacement would provide strong evidence for a role for organizational effects of ovarian hormones on binge eating risk.
Acknowledgements
The authors would like to thank Rayson Figueira for his invaluable help in collecting study data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We conducted post hoc tests to compare PF intake in BEP versus the unclassified, intermediate group of rats to confirm that stable BER/BEP group differences in PF across OVX were not due to our use of extreme groups. In Experiments 1 and 2, the BEP group consumed significantly more PF (1-1.5 grams) than the intermediate group, both before and after OVX, but they did not differ in their chow intake or body weight (data not shown). These findings confirm that results are not due to the use of an extreme BER control group, and that the amount of PF consumed in BEP rats is definitely larger than most rats consume under similar circumstances.
References
- American Psychiatric Association . DSM-IV Sourcebook, Volume 3. American Psychiatric Association; Washington D.C.: 1997. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition - Text Revision (DSM-IV-TR) American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielert C, Busse C. Influences of ovarian hormones on the food intake and feeding of captive and wild female Chacma baboons (Papio ursinus). Physiol. Behav. 1983;30:103–111. doi: 10.1016/0031-9384(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol. Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs. obese binge-eating and obesity with and without binge-eating. International Journal of Obesity (London) 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated startle responsons to opioids in binge-eating rats. Behav. Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- Bulik CM. Eating disorders in adolescents and young adults. Child and Adolescent Psychiatric Clinics. 2002;11:201–218. doi: 10.1016/s1056-4993(01)00004-9. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biol. Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Crow SJ, Keel PK, Thuras P, Mitchell JE. Bulimia symptoms and other risk behaviors during pregnancy in women with bulimia nervosa. Int. J. Eat. Disord. 2004;36:220–223. doi: 10.1002/eat.20031. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Food rejection by female rhesus monkeys during the menstrual cycle and early pregnancy. Physiol. Behav. 1975;14:579–587. doi: 10.1016/0031-9384(75)90185-7. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Ovarian influences on primate food intake: Assessment of progesterone actions. Physiol. Behav. 1978;21:923–928. doi: 10.1016/0031-9384(78)90167-1. [DOI] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: A rhythmic, inhibitory, indirect control of meal size. Physiol. Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol. Med. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Forgie ML, Pellis SM. Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behav. Neurosci. 2004;118:1293–1304. doi: 10.1037/0735-7044.118.6.1293. [DOI] [PubMed] [Google Scholar]
- Garber J, Brooks-Gunn J, Paikoff R, Warne M. Prediction of eating problems: An 8-year study of adolescent girls. Dev. Psychol. 1994;30:823–834. [Google Scholar]
- Gray JM, Greenwood MR. Time course of effects of ovarian hormones on food intake and metabolism. Am. J. Physiol. 1982;243:E407–412. doi: 10.1152/ajpendo.1982.243.5.E407. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress-induced binge eating. Int. J. Eat. Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge-eating: Key synergistic role of past caloric restriction and stress. Physiol. Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Excess mortality of mental disorder. Br. J. Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, Heatherton TF, Dorer DJ, Joiner TE, Zalta AK. Point prevalence of bulimia nervosa in 1982, 1992, and 2002. Psychol. Med. 2005;35:1–9. doi: 10.1017/S0033291705006148. [DOI] [PubMed] [Google Scholar]
- Keel PK, Herzog DB. Long-term outcome, course of illness and mortality in anorexia nervosa, bulimia nervosa, and binge eating disorder. In: Brewerton TD, editor. Eating Disorders. Marcel Dekker; New York: 2004. pp. 97–116. [Google Scholar]
- Kemnitz JW, Eisele SG, Lindsay KA, Engle MJ, Perelman RH, Farrell PM. Changes in food intake during menstrual cycles and pregnancy of normal and diabetic rhesus monkeys. Diabetologia. 1984;26:60–64. doi: 10.1007/BF00252265. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Gibber JR, Lindsey KA, Eisele SG. Effects of ovarian hormones on eating behavior, body weight, and glucoregulation in rhesus monkeys. Horm. Behav. 1989;23:235–250. doi: 10.1016/0018-506x(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Edler C, Keel PK. Ovarian hormones and binge eating: Exploring associations in community samples. Psychol. Med. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk CL, Burt SA. Preliminary evidence that estradiol moderates genetic effects on disordered eating attitudes and behaviors during puberty. Psychol. Med. 2010;40:1745–1754. doi: 10.1017/S0033291709992236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in female adolescent twins. J. Abnorm. Psychol. 2000;109:239–251. [PubMed] [Google Scholar]
- Klump KL, Racine S, Sisk C. Sex differences in binge eating proneness in rats. in preparation.
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. Binge eating proneness emerges during puberty in female rats: A longitudinal study. submitted. [DOI] [PMC free article] [PubMed]
- Lacey JH, Smith G. Bulimia nervosa. Br. J. Psychiatry. 1987;150:777–781. doi: 10.1192/bjp.150.6.777. [DOI] [PubMed] [Google Scholar]
- Marrone BL, Roy EJ, Wade GN. Progesterone stimulates running wheel activity in adrenalectomized-ovariectomized rats. Horm. Behav. 1975;6:231–236. doi: 10.1016/0018-506x(75)90010-0. [DOI] [PubMed] [Google Scholar]
- Morgan JF, Lacey JH, Sedgwick P. Impact of pregnancy on bulimia nervosa. Br. J. Psychiatry. 1999;174:135–140. doi: 10.1192/bjp.174.2.135. [DOI] [PubMed] [Google Scholar]
- NIMH . Eating Disorders. Rockville, MD: 1994. NIH publication No. 94-3477. [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int. J. Eat. Disord. doi: 10.1002/eat.20808. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Tambs K, Harris JR. Genetic and environmental influences on binge eating in the absence of compensatory behaviors: A population-based twin study. Int. J. Eat. Disord. 2004;36:307–314. doi: 10.1002/eat.20047. [DOI] [PubMed] [Google Scholar]
- Rosenblatt H, Dyrenfurth I, Ferin M, Vande Weile RL. Food intake and menstrual cycle in Rhesus monkeys. Physiol. Behav. 1980;24:447–449. doi: 10.1016/0031-9384(80)90234-6. [DOI] [PubMed] [Google Scholar]
- Roth ME, Negus SS, Knudson IM, Burgess MP, Mello NK. Effects of gender and menstrual cycle phase on food-maintained responding under a progressive-ratio schedule in cynomolgus monkeys. Pharmacol. Biochem. Behav. 2005;82:735–743. doi: 10.1016/j.pbb.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira PP, Portella AK, Crema L, Correa M, Nieto FB, Diehl AB, Lucion AB, Dalmaz C. Both infantile stimulation and exposure to sweet food lead to an increased sweet food ingestion in adult life. Physiol. Behav. 2008;93:877–882. doi: 10.1016/j.physbeh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Puberty hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Stice E, Cameron RP, Killen JD, Hayward C, Taylor CB. Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. J. Consult. Clin. Psychol. 1999:967–974. doi: 10.1037//0022-006x.67.6.967. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: A 2-year prospective investigation. Health Psychol. 2002;21:131–138. [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm. Behav. 2008;54:471–477. doi: 10.1016/j.yhbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, Blaha V. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol. Behav. 1999;68:99–107. doi: 10.1016/s0031-9384(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol. Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J. Comp. Physiol. Psychol. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- Wade TD, Bergin JL, M. T, Bulik CM, Fairburn CG. Prevalence and long-term course of lifetime eating disorders in an adult Australian twin cohort. Aust. N. Z. J. Psychiatry. 2006;40:121–128. doi: 10.1080/j.1440-1614.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- Willis DC, Rand CS. Pregnancy in bulimic women. Obstet. Gynecol. 1988;71:708–710. [PubMed] [Google Scholar]
- Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiol. Behav. 2008;95:501–507. doi: 10.1016/j.physbeh.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, Klump KL. Long-term effects of early puberty on disordered eating and anxiety in men and women. Horm. Behav. 2007;52:427–435. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]