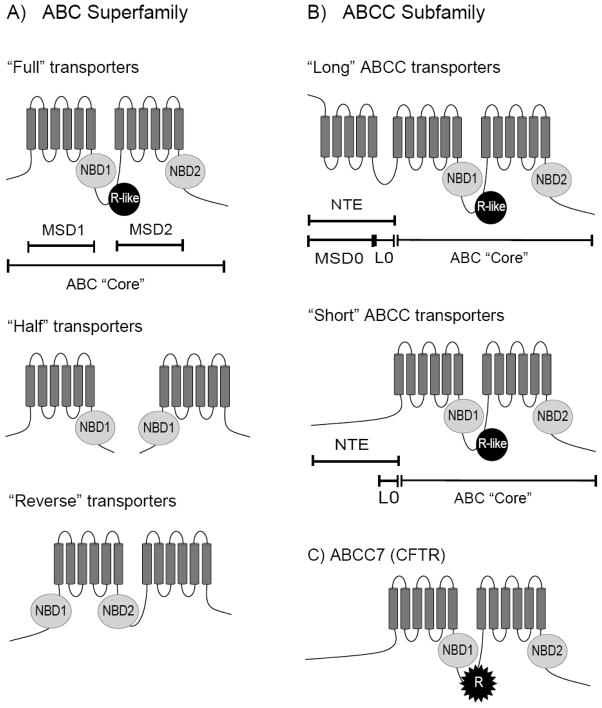
Fig. 1. Predicted topology of ATP-Binding Cassette (ABC) transporters.
(A) In general, ABC transporters are structurally characterized as having an ABC “Core” containing two membrane spanning domains (MSDs), also called transmembrane spanning domains (TMDs), and two intracellular nucleotide binding domains (NBDs). A number of ABC transporters exist as “Half” transporters which form homo- and/or heterodimers. (B) The ABCC subfamily has N-terminal extension (NTE) in addition to the ABC “Core”, The NTE of “Long” ABCC proteins consists of an extra membrane spanning domain (MSD0, TMD0) and an extra linker domain (L0) (also called cytoplasmic loop 3 (CL3)). In “Short” ABCC proteins, the MSD0 is absent. (C) The linker region between NBD1 and MSD2 of ABCC7 (CFTR) is extensively phosphorylated and is thus referred to as the regulatory domain (R domain). Similar domains are found in other ABC transporters and are referred to here as the “R-like” domain (annotated in Fig. 1A).