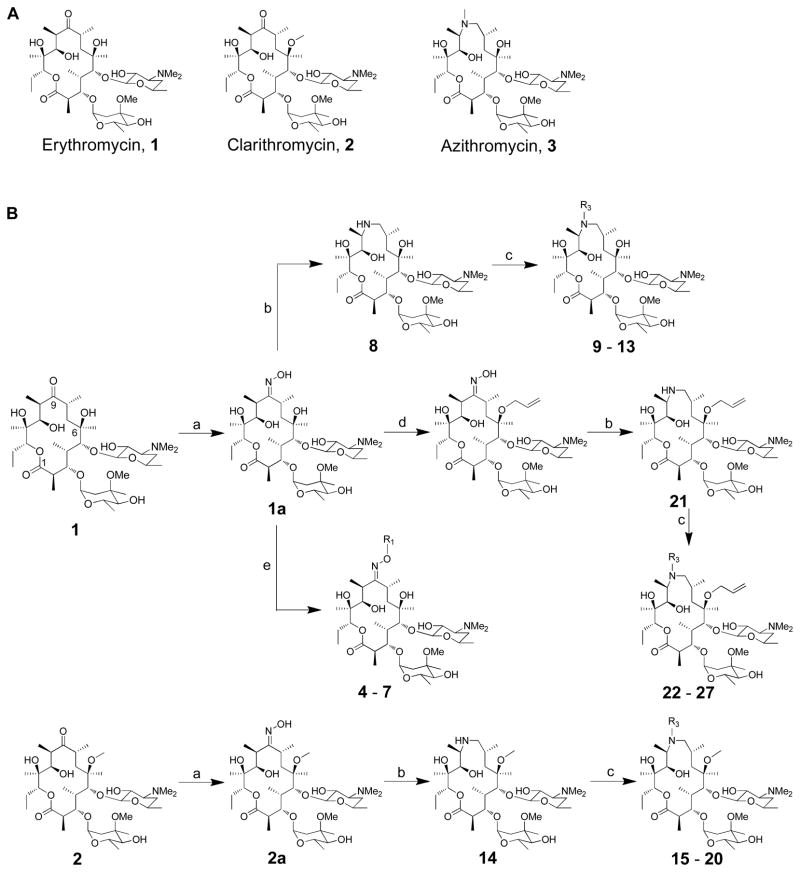
Figure 1.
(A) Structures of erythromycin, clarithromycin and azithromycin; (B) Synthesis of erythromycin A oxime ethers (4 – 7) and 15-membered azithromycin analogues (8 – 31). (a) 50% NH2OH (aq.), AcOH in iso-propanol; (b) i - toluene-ρ-sulphonyl chloride in MeOH, ii – NaBH4 in MeOH; (c) corresponding aldehyde, AcOH, NaBH3CN in DMF; (d) i - pyridine·HCl, cyclohexane diethyl ketal in CH3CN, ii – pyridine·HCl, hexamethyldisilazane in CH3CN, iii – allyl tert-butyl carbonate, Pd2(dba)3, dppb in THF, iv – AcOH in CH3CN and H2O; (e) corresponding alkyl halides, 1M potassium tert-butoxide in THF;