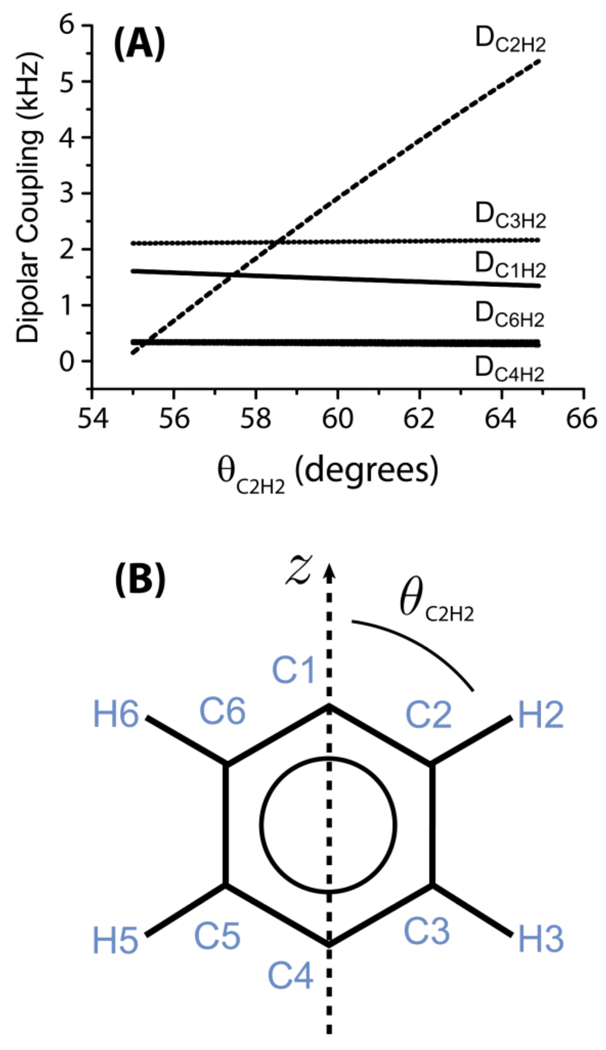
Figure 2. Dependence of heteronuclear dipolar coupling on the molecular orientation.
The dipolar couplings between proton-2 (H2) and nearby carbons in a aromatic ring of a liquid crystal. (A) The dipolar couplings between H2 and the various carbons as a function of θC2H2 (refer to part B). (B) The phenyl moiety of a liquid crystal. It connects to the rest of the liquid crystal molecule at C4 and/or C1; an example, the molecular structure of MBBA liquid crystal is given in Figure 5. θC2H2 defines the orientation of the C2-H2 bond relative to the z-axis, which is the director axis for the ring-flip motions of the phenyl moiety. The z-axis also defines the direction of the magnetic field of NMR spectrometer.