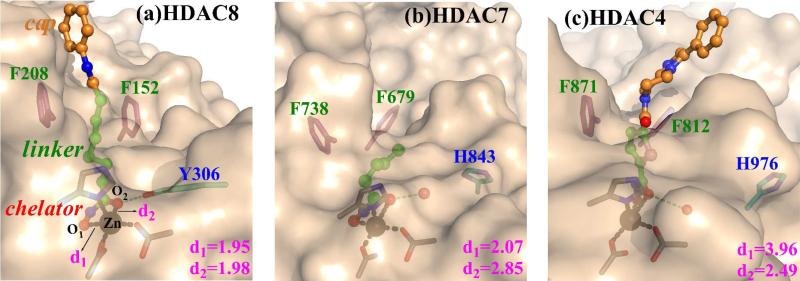
Figure 1.
The active site of enzyme-inhibitor complex in HDAC8 (a), HDAC7 (b) and HDAC4 (c). For HDAC8, the inhibitor is SAHA, for HDAC7, it is a truncated-SAHA in the crystal structure, and for HDAC4, it is a SAHA-like hydroxamic Acid (HA). The conserved two phenylalanines are located at the entrance of the pocket. Y306 forms a hydrogen bond with SAHA in HDAC8 but it is replaced by a His in HDAC7/4, in which a crystal water is close enough to form the hydrogen bond with the inhibitor. The oxygen-zinc distances d1/d2 were measured from XRD structures.16,19,20