Abstract
Viperid snakes of the genus Atropoides are distributed in Mexico and Central America and, owing to their size and venom yield, are capable of provoking severe envenomings in humans. This study evaluated, using an ‘antivenomics’ approach, the ability of a polyspecific (polyvalent) antivenom manufactured in Costa Rica to recognize the proteins of Atropoides mexicanus and A. picadoi venoms, which are not included in the immunization mixture. In addition, the neutralization of lethal, hemorrhagic, myotoxic, coagulant, proteinase and phospholipase A2 (PLA2) activities of these venoms by the antivenom was assessed. The antivenom was highly-effective in immunodepleting many venom components, particularly high molecular mass P-III metalloproteinases (SVMPs), L-amino acid oxidases, and some serine proteinases and P-I SVMPs. In contrast, PLA2s, certain serine proteinases and P-I SVMPs, and a C type lectin-like protein were only partially immunodepleted, and two PLA2 molecules were not depleted at all. The antivenom was able to neutralize all toxic and enzymatic activities tested, although neutralization of lethality by A. nummifer venom was achieved when a challenge dose of 3 LD50s of venom was used, but was iffective when 4 LD50s were used. These results, and previously obtained evidence on the immunoreactivity of this antivenom towards homologous and heterologous venoms, revealed the low immunogenicity of a number of venom components (PLA2s, CRISPs, P-I SVMPs, and some serine proteinases), underscoring the need to search for innovative immunization protocols to improve the immune response to these antigens.
Keywords: Atropoides picadoi, Atropoides mexicanus, antivenomics, antivenom, neutralization, toxicity, immunodepletion, snake venom
INTRODUCTION
Animal-derived antivenoms constitute the cornerstone in the treatment of envenomings by snakebites worldwide (WHO, 2010). In contrast to other antibody-based immunotherapeutics, such as various antitoxins and rabies immunoglobulin, the antigens (snake venoms) used for the preparation of snake antivenoms exhibit a large intra- and inter-species variability (Chippaux et al, 1991; Alape-Girón et al, 2008; Calvete et al, 2010b). Consequently, the selection of the venoms to be used in the immunizing mixtures to raise snake antivenoms is a key issue in the design of these immunobiologicals (Gutiérrez et al, 2009a; WHO, 2010). Antivenoms can be either monospecific, if venom of only one species is used for immunization, or polyspecific, when animals are immunized with venoms from two or more species (Theakston et al, 2003; WHO, 2010). In either case, the generated antibodies recognize not only proteins present in the homologous venoms, but also antigenically-related proteins present in heterologous venoms of phylogenetically-related species. Such paraspecificity may in turn result in effective neutralization of venoms not included in the immunizing mixture, as has been shown in the case of Bothrops antivenoms in Latin America (Segura et al, 2010). However, there are also situations in which antivenoms fail to neutralize venoms of closely related species, as has been documented for neotropical rattlesnakes (Saravia et al, 2002; Calvete et al, 2010b). Therefore, the detailed analysis of the paraspecific neutralization and immunoreactivity of antivenoms against venoms of medically-relevant species is a necessary task for establishing their preclinical spectrum of efficacy.
The family Viperidae comprises 23 snake species in Central America (Campbell and Lamar, 2004), some of which are responsible for the vast majority of snakebites in this region (Gutiérrez, 2009). These species are classified within the genera Agkistrodon, Atropoides Bothriechis, Bothrops, Cerrophidion, Crotalus, Lachesis and Porthidium (Campbell and Lamar, 2004). The genus Atropoides includes a number of thick-bodied species, known as jumping vipers, distributed in Mexico and Central America (Campbell and Lamar, 2004). Although very little information is available regarding the incidence of snakebites caused by these species to humans, it is likely that they inflict a number of accidents due to their broad distribution and relative abundance. In addition, the similarity of clinical symptoms with those caused by other pitvipers, such as Bothrops asper, makes the identification of Atropoides bites rather difficult. Despite the lack of epidemiological records on bites by these species, their size and relatively high venom yield (Bolaños, 1972) suggests that they may be able to provoke significant envenomings in humans.
A polyspecific antivenom, prepared by immunizing horses with the venoms of Bothrops asper, Crotalus simus and Lachesis stenophrys, is widely used in Central America for the treatment of envenomations caused by species of the family Viperidae (Angulo et al, 1997; Gutiérrez, 2009). Early on after the development of this antivenom, its ability to neutralize the lethal effect of viperid venoms from Costa Rica, including those of Atropoides species, was demonstrated (Bolaños, 1971). Further studies evaluated the neutralization of proteolytic, hemorrhagic, indirect hemolytic, edema-forming, coagulant and defibrinating activities of Central American snake venoms by this antivenom, including the venoms of Atropoides nummifer (mexicanus) and A. picadoi (Gutiérrez et al, 1985, 1986; Rojas et al, 1987, 2001; Gené et al, 1989; Valiente et al, 1992). Recently, proteomic analytical tools have been adapted for the analysis of the immunoreactivity of antivenoms against venoms, a field of study coined ‘antivenomics’ (Lomonte et al, 2008; Gutiérrez et al, 2009a; Calvete et al, 2009a; Calvete, 2010). Once the proteomic profile of a particular venom (the ‘venome’) is deciphered, then the immunoreactivity of antivenoms against the different venom components can be investigated, thus allowing a detailed assessment of the immune recognition scope of antivenoms. This information, together with the analysis of the neutralizing ability against specific toxicological and enzymatic effects of venoms, provides a comprehensive view of the cross-reactivity and neutralization spectrum of antivenoms against homologous and heterologous venoms. In turn, this information can be used for a more rigorous design of immunizing mixtures for the manufacture of more effective antivenoms.
The venomes of two species of Atropoides snakes, A. nummifer (mexicanus) and A. picadoi, from Costa Rica have been recently reported (Angulo et al, 2008). Despite the close phylogenetic kinship of these two species, their venoms comprise rather dissimilar proteomes, with phospholipases A2 (PLA2s) being the major toxins present in A. mexicanus venom, whereas Zn2+-dependent metalloproteinases (SVMPs) predominate in A. picadoi venom (Angulo et al, 2008). Such proteomic profiles are consistent with the pathophysiological alterations induced by A. mexicanus and A. picadoi venoms in mice, as the former induces prominent PLA2-mediated myonecrosis while the latter causes SVMP-mediated hemorrhage, being the venom with the highest hemorrhagic potential among Costa Rican viperids (Gutiérrez et al, 1985). The present work is aimed at describing a detailed antivenomic assessment of the immunoreactivity against the venoms of A. mexicanus and A. picadoi of the polyspecific antivenom used in Central America. In addition, the neutralization of the most relevant toxic and enzymatic activities of A. mexicanus and A. picadoi venoms by the antivenom was also investigated. The results evidenced a conspicuous pattern of cross-reactivity and paraspecific protection of the antivenom, but also identified a number of venom components against which the antivenom has a weak antibody repertoire, thus providing useful information for the improvement of this immunotherapeutic.
MATERIALS AND METHODS
Venoms and antivenom
Venoms were obtained from at least 20 adult specimens of each species collected in Costa Rica and kept at the serpentarium of Instituto Clodomiro Picado (ICP). Venoms were freeze-dried immediately after collection, and stored at -20ºC. Polyspecific (polyvalent, Crotalinae) antivenom (Batch 420, expiry date October 1st, 2010) from ICP was used. This antivenom is routinely prepared at ICP from the plasma of horses immunized with a mixture of the venoms of Bothrops asper, Crotalus simus and Lachesis stenophrys (Angulo et al, 1997), and consists of immunoglobulins purified by caprylic acid precipitation (Rojas et al, 1994). A control preparation of normal equine IgG was prepared by an identical fractionation of the plasma of horses which had not been immunized with snake venoms.
Antivenomics: Immunodepletion of venom proteins by the ICP polyvalent antivenom
We have coined the term "antivenomics" for the proteomic characterization of venom proteins bearing epitopes recognized by an antivenom (Lomonte et al, 2008; Calvete et al, 2009a and 2009b; Gutiérrez et al, 2009a). Briefly, two milligrams of whole venom were dissolved in 70µl of 20mM phosphate buffer, pH 7.0, and mixed with 8mg of polyvalent antivenom IgGs overnight at room temperature (∼22ºC). IgG concentration was determined spectrophotometrically at 280nm using an extinction coefficient (ε) of 1.4 for a 1mg/ml protein concentration determined in a 1cm light path length cuvette (Fasman, 1992). Thereafter, the reaction mixture was incubated for 1hr at 37ºC with 400μl of Protein G-Sepharose (nominal binding capacity of 20mg IgG/ml) (GE-Healthcare) in 20mM phosphate buffer, pH 7.0. Protein G-bound immunocomplexes were sedimented at 3,000rpm for 10min in an Eppendorf microcentrifuge, and the supernatant was submitted to reverse-phase HPLC separation using an ETTANTM LC HPLC system (Amersham Biosciences) and a Lichrosphere RP100 C18 column (250x4mm, 5µm particle size) developed at 1ml/min with a linear gradient of 0.1% (w/v) TFA in water (solution A) and acetonitrile (solution B) (5%B for 10min, followed by 5-15%B over 20min, 15-45%B over 120min, and 45-70%B over 20min) (Angulo et al, 2008). Protein detection was at 215nm and peaks were collected manually and dried in a Speed-Vac (Savant). HPLC-fractions were assigned to previously characterized toxins (Angulo et al, 2008) by their HPLC elution profile, N-terminal sequencing (using a Procise instrument, Applied Biosystems, Foster City, CA, USA) and SDS-PAGE. Control samples were subjected to the same procedure except that control equine IgGs, instead of antivenom IgGs, were included in the reaction mixture. Quantitation of immunodepletion was assessed by comparing the peak areas of control and treated samples. Depending on the extent of immunodepletion, venom proteins were categorized as: C-toxins, corresponding to completely immunodepleted proteins; P-toxins, corresponding to partially immunodepleted proteins; and N-toxins, non-immunodepleted proteins (Calvete et al, 2009b).
Antivenomics: Immunoblot analysis
The presence of polyvalent antivenom antibodies directed against antigenic determinants of A. mexicanus and A. picadoi venom proteins was also investigated by Western Blot analysis. To this end, the reverse-phase HPLC chromatographic fractions were separated by SDS-PAGE (15%, w/v, polyacrylamide concentration) under non-reducing conditions, followed by electrotransfer to nitrocellulose membranes (Towbin et al, 1979) using a Bio-Rad minitransfer cell operated at 150mA during 90min. To assess transfer efficiency, nitrocellulose membranes were visualized by reversible Ponceau-S Red staining. Then, the membranes were destained by exhaustive washing with distilled water, and incubated in 2% (w/v) bovine serum albumin in phosphate-buffered saline solution (PBS; 0.12M NaCl, 0.04M sodium phosphate, pH 7.2) for 30min at room temperature to block non-specific binding sites, followed by incubation for 90min with 1:3,000 dilution (in PBS) of antivenom or control equine IgGs. After washing four times with PBS containing 0.2% (w/v) albumin and 0.05% (v/v) Tween-20, the membranes were incubated for 90min with anti-horse IgG-alkaline phosphatase conjugate (Sigma) diluted 1:6,000. Finally, the membranes were washed five times as above, and color development was performed by adding BCIP/NBT (Chemicon) substrate.
Neutralization of toxic and enzymatic activities
The toxic and enzymatic activities of the venoms were assessed using previously described protocols. Briefly, lethality was determined by intraperitoneal injection in CD-1 mice (16-18gm) (Bolaños, 1972). Hemorrhage was assessed by the intradermal test in mice (Gutiérrez et al, 1985). The Minimmum Hemorrhagic Dose (MHD) was estimated as the amount of venom inducing a hemorrhagic halo of 10mm in the skin 2hr after injection. Myotoxicity was determined in mice (18-20gm) injected intramuscularly, in the right gastrocnemius, with solutions of varying venom concentration (Instituto Clodomiro Picado, 2008). Three hours after injection, mice were bled and the plasma creatine kinase (CK; E.C. 2.7.3.2) activity was determined by means of a kinetic assay (CK-Nac, Biocon Diagnostik, Germany). The Minimum Myotoxic Dose (MMD) was defined as the amount of venom that induced an increment in plasma CK activity corresponding to 4 times the value in control mice injected with PBS alone. Coagulant activity was determined in human citrated plasma (Theakston and Reid, 1983; Gené et al, 1989). The Minimum Coagulant Dose (MCD) corresponds to the amount of venom that induces clotting of plasma in 60sec. Proteolytic activity was determined using azocasein as substrate (Wang et al, 2004), as modified by Gutiérrez et al (2008). One unit of proteolytic activity corresponds to the amount of venom that induces a change in absorbance at 450nm of 0.2. PLA2 activity was determined titrimetrically using egg yolk phospholipids as substrate (Dole, 1956; Gutiérrez et al, 1986). Activity was expressed as μEq fatty acid released per mg protein per min. All experiments involving the use of animals were approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUA) of Universidad de Costa Rica.
Neutralization of venom activities by the polyvalent antivenom was assessed by using a previously described protocol based on the incubation of a fixed dose of venom (‘challenge dose’) with various dilutions of antivenom, for 30min at 37ºC, in order to achieve various ratios of μl antivenom/mg venom (Gutiérrez et al, 1990; Instituto Clodomiro Picado, 2008). Then, aliquots of the mixtures, containing a challenge dose of venom, were tested in the corresponding experimental systems described above. Controls included venom solutions incubated without antivenom. The challenge doses of venom used were; lethality, 3 or 4 LD50; hemorrhagic activity, 10 or 5 MHD; myotoxic activity, 3 MMD in the case of A. picadoi and 5 MMD in the case of A. nummifer; coagulant activity, 3 MCDs; proteolytic activity, 10μg for both venoms; and PLA2 activity, 80μg venom for A. nummifer and 40μg for A. picadoi. The challenge doses for proteolytic and PLA2 activities were selected from dose-response curves and corresponded to doses exerting submaximal activity. Neutralization was expressed as Effective Dose 50% (ED50), corresponding to the ratio μl antivenom/mg venom, which reduced by 50% the effect induced by the venom (Gutiérrez et al, 1990). In the case of coagulant activity, neutralization was expressed as Effective Dose (ED), corresponding to the ratio μl antivenom/mg venom in which the clotting time of plasma was prolonged three times when compared with the clotting time of plasma incubated with venom alone.
RESULTS
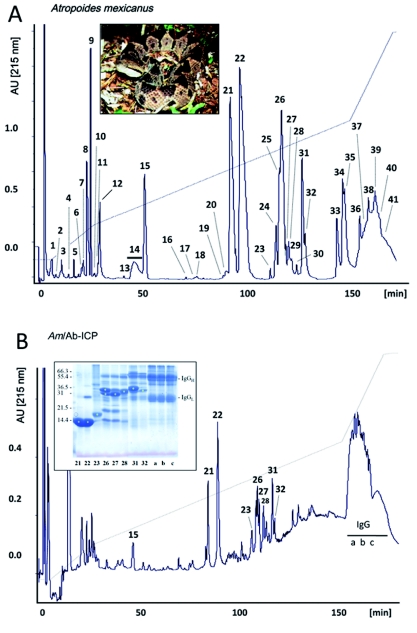
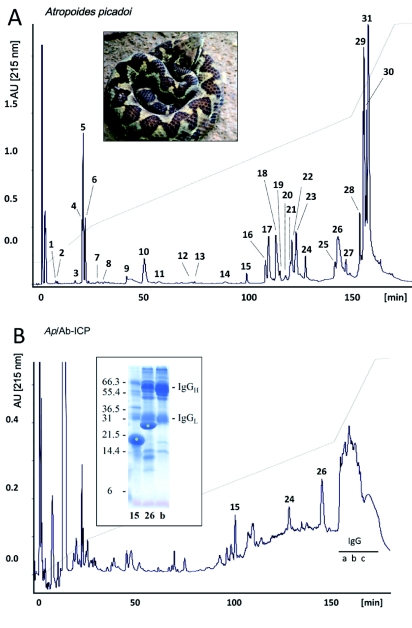
Antivenomics
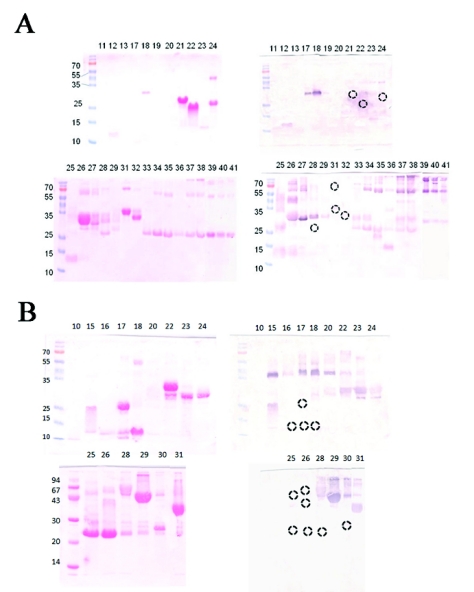
Figures 1 and 2, and Table 1 present the results of the immunodepletion of venom proteins by the antivenom. The antivenom completely immunodepleted various proteins of A. mexicanus venom (C-toxins), immunodepleted 60-70% of the major PLA2s 21 and 22, and 75-80% of serine proteinases 26, 27, 28, 31 and 32 (P-toxins), and did not reduce the peak of PLA2 23 (N-toxin) (Figure 1 and Table 1). On the other hand, most proteins were quantitatively immunodepleted from the venom of A. picadoi by the antivenom (Figure 2 and Table 1), thus corresponding to C-toxins. However, proteins 24 (a serine proteinase) and 26 (a P-I SVMP) were only partially (∼40%) depleted from the venom, thus corresponding to P-toxins (Table 1). Notably, PLA2 15 was not immunoprecipitated at all (N-toxin). Immunoblot analysis was in line with most of the results obtained by the immunodepletion approach (Figure 3). In the case of A. mexicanus venom, N- and P-toxins were also poorly recognized by Western blotting, with the exception of fractions 26 and 27 (serine proteinases). A further discrepancy between immunoblotting and immunodepletion in A. mexicanus venom was on protein 24 (a cysteine-rich secretory protein, CRISP), which was not recognized by the antivenom in Western blot but was partially immunodepleted in the antivenomic assay. Proteins from the venom of A. picadoi that were not recognized by immunoblot were proteins 16 (PLA2), 17 (CRISP), 18 (PLA2), a P-I SVMP present in fractions 25, 26 and 28, and a C type lectin-like protein eluted in fraction 30 (Figure 3 and Table 1). In this venom, a number of proteins that were immunodepleted by the antivenom failed to be recognized by immunoblotting (Table 1). On the other hand, PLA2 15 and serine proteinase 24 of A. picadoi venom were not immunodepleted, but were recognized by antivenom IgGs in western blot (Table 1).
Figure 1.
Reverse-phase HPLC separation of proteins from control (A) and polyvalent antivenom-immunodepleted (B) Atropoides mexicanus venom. Protein numbering and identity correspond to those in Table 1 of Angulo et al (2008). Letters correspond to fractions containing IgG molecules. The inserts show a photo of an adult A. picadoi specimen and a SDS-PAGE analysis of the β-mercaptoethanol-reduced fractions isolated by reverse-phase HPLC. Venom proteins are labeled with asterisks.
Figure 2.
Reverse-phase HPLC separation of proteins from control (A) and polyvalent antivenom-immunodepleted (B) Atropoides picadoi venom. Protein numbering and identity corresponds to those in Table 2 of Angulo et al (2008). Letters correspond to fractions containing IgG molecules. The insert shows a photo of an adult A. mexicanus specimen and a SDS-PAGE of the β-mercaptoethanol-reduced fractions isolated by reverse-phase HPLC. Venom proteins are labeled with asterisks.
Table 1.
Proteins from the venoms of A. mexicanus and A. picadoi that were only partially immunodepleted or not recognized by immunoblotting with polyvalent antivenom. Numbering of fractions corresponds to those of Figures 1 and 2.
| Species | Fractions | Molecular mass (Da) | Techniquea | Protein Family |
|---|---|---|---|---|
| A. mexicanus | 21 | 13,751 | WB and IP | PLA2 (Myotoxin I) |
| 22 | 13,792 | WB and IP | PLA2 (Myotoxin II) | |
| 24 | 24,744 | WB | CRISP | |
| 26 | 30,000 | IP | Serine proteinase | |
| 27 27 |
26,000 33,000 |
IP IP |
Serine proteinase Serine proteinase |
|
| 28 | 26,000 | WB and IP | Serine proteinase | |
| 31 | 34,000 | WB and IP | Serine proteinase | |
| 32 | 34,000 | WB and IP | Serine proteinase | |
| A. picadoi | 15 | 13,897.6 | IP | PLA2 |
| 16 | 13,806.6 | WB | PLA2 | |
| 17 | 24,787.1 | WB | CRISP | |
| 18 | 13,790.6 | WB | PLA2 | |
| 24 | 28,000 | IP | Serine proteinase | |
| 25 | 22,900 | WB | P-I SVMP | |
| 26 | 22,900 | WB and IP | P-I SVMP | |
| 28 | 22,900 | WB | P-I SVMP | |
| 30 | 28,000 | WB | C type lectin-like |
aTechnique by which immunoreactivity was assessed: Western Blot (WB) or immunoprecipitation (IP).
Figure 3.
Antivenomic profile of A. mexicanus (A) and A. picadoi (B) snake venoms by western blot analysis. Venom fractions isolated by HPLC were then separated by SDS-PAGE under non-reducing conditions, and transferred to nitrocellulose membranes. Ponceau-S Red reversible staining of transferred RP-HPLC fractions is shown at left and western blot of the fractions is shown at right. Lane numbers correspond to peaks in HPLC chromatography, and migration of molecular mass markers is depicted to the left. Circles are used to highlight protein bands not recognized by the polyvalent antivenom. Some bands not recognized by the antivenom were not included in Table 1 because their identity has not been determined in proteomic analysis.
Neutralization of toxic and enzymatic activities
Table 2 displays the toxic and enzymatic activities of the venoms of A. mexicanus and A. picadoi. In the case of proteolytic activity on azocasein, it largely depends on the action of SVMPs, since incubation of venoms with 20 mM EDTA impaired by > 95% the activity of both venoms. On the basis of these activities, the challenge doses described in the Materials and Methods section were selected. The polyvalent antivenom was effective in the neutralization of the toxic and enzymatic activities of the two Atropoides venoms (Table 3). Concerning the lethal activity of A. mexicanus venom, the antivenom failed to neutralize this effect when a challenge dose of 4 LD50s was used. However, the antivenom neutralized lethality when the challenge dose was 3 LD50s. In contrast, the lethal activity of A. picadoi venom was readily neutralized by the antivenom even when using a challenge dose of 4 LD50s (Table 3). Neutralization of hemorrhagic and PLA2 activities required, respectively, the least and the highest volume of antivenom (Table 3). For comparative purposes, the neutralizing ability of this antivenom against the toxic and enzymatic effects of Bothrops asper venom, which is used in the immunizing mixture for the preparation of this antivenom, is also shown in Table 3.
Table 2.
Toxic and enzymatic activities of the venoms of A. nummifer and A. picadoi.
| Activitya | A. mexicanus | A. picadoi |
|---|---|---|
| Lethality (LD50) | 125μg | 42μg |
| Hemorrhagic (MHD) | 6μg | 0.25μg |
| Myotoxic (MMD) | 4.2µg | 13µg |
| Coagulant (MCD) | 15μg | No coagulation at 50μg |
| Proteolytic (units/mg) | 150 | 145 |
| Phospholipase A2 (μEq/mg/min) | 4.6 | 11.6 |
aToxic activities are expressed as Median Lethal Dose (LD50), Minimum Hemorrhagic Dose (MHD), Minimum Myotoxic Dose (MMD) and Minimum Coagulant Dose (MCD) (See Materials and Methods for details).
Table 3.
Neutralization of toxic and enzymatic activities of A. mexicanus and A. picadoi venoms by the polyvalent antivenom from Instituto Clodomiro Picado.
| Neutralization (ED50)a | |||
|---|---|---|---|
| Venom activity | mexicanus | picadoi | Bothrops asperb |
| Lethality (4 LD50s) | >2000µl/mg | 353 (249-500) µl/mg | 342 (224-465) |
| Lethality (3 LD50s) | 891 (565-1404) µl/mg | Not tested | Not tested |
| Hemorrhagic | 112 ± 27µl/mg | 48 ± 38µl/mg | 175 ± 37µl/mg |
| Myotoxic | 1134 ± 275 | 380 ± 174 | 555 ± 159 |
| Coagulant | 972 ± 36µl/mg | Venom devoid of coagulant effect | 133 ± 8µl/mg |
| Proteolytic | 1394 ± 44µl/mg | 1481 ± 36µl/mg | 942 ± 22µl/mg |
| Phospholipase A2 | 402 ± 16µl/mg | 901 ± 69µl/mg | 1268 ± 182µl/mg |
aNeutralization is expressed as the Effective Dose 50% (ED50) corresponding to the ratio µL antivenom/mg venom at which the effect of the challenge dose of venom was reduced to 50%. In the case of coagulant effect, neutralization is expressed as Effective Dose (ED), corresponding to the ratio µL antivenom/mg venom at which the clotting time of plasma is prolonged three times as compared with clotting time of plasma incubated with venom alone (see Materials and methods for details). In the case of lethality, the 95% confidence limits are given in parentheses. For the other effects, results are presented as mean ± S.D. (n = 4).
bResults of neutralization of B. asper venom correspond to those described by Saravia et al (2001) using similar methodologies. In the case of proteolytic activity, ED50 was reported by Gutiérrez et al (2010).
DISCUSSION
Preclinical assessment of the neutralizing ability of antivenoms has been traditionally based on the neutralization of the lethal activity of venoms in mice (WHO, 1981). Although the mouse lethality test still remains as the gold standard for antivenom potency estimation (WHO, 2010), the biochemical and toxicological complexity of snake venoms urges a more comprehensive analysis of antivenom preclinical efficacy. A relevant step forward in this area was the introduction of neutralization tests based on specific toxic and enzymatic venom activities besides lethality (Theakston, 1986; Gutiérrez et al, 1996; Instituto Clodomiro Picado, 2008). In the case of viperid snake venoms, the neutralization of hemorrhagic, coagulant, defibrinogenating and myotoxic activities are of particular relevance. Recently, a novel approach in the assessment of antivenom immunoreactivity, termed ‘antivenomics’, has been developed (Lomonte et al, 2008; Gutiérrez et al, 2009a; Calvete et al, 2009a). The antivenomic approach is based on a proteomic methodological platform, which permits the identification of all proteins present in venoms, allowing therefore the analysis of the immunoreactivity of an antivenom against each individual component in a venom. Immunoblotting techniques have been also used for identifying venom proteins recognized by antivenom antibodies (Lomonte et al, 2008; Correa-Netto et al, 2010). The combination of immunodepletion, immunoblotting, and neutralization approaches allows a detailed evaluation of the preclinical efficacy of antivenoms. Here we use this integrated methodology to uncover the paraspecific reactivity and neutralization ability of a polyvalent antivenom against the venoms of two species of Atropoides from Central America.
A modification of the original antivenomics protocol was introduced for this work. Instead of precipitating venom-antivenom complexes by the addition of anti-horse IgG, as originally described by Lomonte et al (2008), the immune complexes were removed by adsorption to protein G-Sepharose beads, which bind horse IgG Fc domains, thereby allowing their depletion from the reaction mixture by centrifugation. This modification greatly reduces the concentration of free anti-horse IgG molecules in the supernatant, thus simplifying the reverse-phase chromatographic separation. As in previous antivenomic studies (Lomonte et al, 2008; Gutiérrez et al, 2008; 2010), this approach was combined with western blot analysis of antivenom reactivity. In general, immunoprecipitation and immunoblotting analyses had a good correlation, with some exceptions. Such discrepancies may be based on the fact that immunoblotting is performed on denatured proteins, which may affect some conformational epitopes in venom components. The antivenom recognized most of the proteins in the two Atropoides venoms by both antivenomic immunodepletion and immunoblotting. However, a number of toxins were immunodepleted only to a partial extent. In the case of A. mexicanus venom, P-toxins included PLA2s, CRISP, and various serine proteinases. Similarly, the A. picadoi venom's P-toxins comprised PLA2s, CRISP, a serine proteinase, a P-I SVMP and a C-type lectin-like protein. There were two PLA2s – one in each venom – which were not recognized by the antivenom, being therefore N-toxins. The toxic profile of these poorly recognized proteins remains unknown. Our findings resemble previous results obtained with the venoms of Bothrops asper (Gutiérrez et al, 2010), B. caribbaeus and B. lanceolatus (Gutiérrez et al, 2008), Bothriechis lateralis and B. schlegelii (Lomonte et al, 2008). In these cases, high molecular mass venom proteins, such as P-III SVMPs and L- amino acid oxidases, were completely immunodepleted by the antivenom (C-toxins), whereas proteins of lower molecular mass, especially disintegrins, CRISPs and PLA2s were immunodepleted only to a partial extent (P-toxins) or, in some cases, not depleted at all (N-toxins). Other components of intermediate molecular mass, such as serine proteinases, P-I SVMPs and C-type lectin-like proteins showed an intermediate pattern of immunodepletion. The fact that a clear relationship exists between molecular mass of venom proteins and their immunorecognition by antivenom IgGs regardless of their relative abundance in the venoms is not surprising, since the surface area accessible to the immune system is directly proportional to the molecular mass of the protein. Hence, the contact area of antigen and antibody varies between 600 and 1200 A2 and involves about 14-21 surface-exposed residues (Padlan et al, 1989), and thus a small protein binds simultaneously less IgG molecules than a higher molecular mass protein. Interestingly, the low immunoreactivity of the polyvalent antivenom against a number of Atropoides venom toxins appears not to depend on the fact that these venoms were not included in the immunizing mixture, since a highly similar pattern of immunodepletion was observed against the venom of B. asper (Gutiérrez et al, 2010), which is included in the venom mixture used in immunization (Angulo et al, 1997).
Neutralization studies confirmed the efficacy of the polyvalent antivenom to neutralize the toxic and the enzymatic activities of the two Atropoides venoms, albeit in the case of lethality induced by A. mexicanus venom, such neutralization was achieved only when a challenge dose of up to 3 LD50s was used. Our findings agree with previous studies on the neutralization of Atropoides venoms by this polyspecific antivenom, although quantitative differences in the values of ED50s have been reported, probably evidencing variations between different batches of antivenom or intraspecies variability in the composition of venoms from various geographical origins (Gutiérrez et al, 1985; Rojas et al, 1987; 2001; Gené et al, 1989; Bogarín et al, 2000). As in the case of immunodepletion experiments, the ED50 values for the neutralization of toxic and enzymatic activities of these heterologous venoms were similar to those for the neutralization of B. asper venom, which is part of the immunizing mixture (Saravia et al, 2001; Gutiérrez et al, 2010; and Table 3). Moreover, in the case of neutralization of PLA2 activity, antivenom was more effective against heterologous Atropoides venoms than against homologous B. asper venom (Table 3). This further supports the concept that there is a significant antigenic similarity between the main toxins in B. asper and Atropoides venoms, and that the low immunoreactivity against some venom components in Atropoides is not due to the heterologous nature of these venoms but, instead, to the low immunogenicity of some venom components, i.e., PLA2s, CRISPs, and some P-I SVMPs and serine proteinases.
The proteomic differences between the venoms of A. mexicanus and A. picadoi have evident implications in the neutralization by antivenom. P-III SVMPs comprise 56% of the venom of A. picadoi (Angulo et al, 2008). P-III SVMPs are mostly responsible for the hemorrhagic activity of venoms (Gutiérrez et al, 2005) and, in the case of A. picadoi, it is likely that P-III SVMPs constitute major lethal factors and also toxins responsible for local myotoxicity, since this venom has a low content of myotoxic PLA2s (Angulo et al, 2008); consequently, local myonecrosis is mostly secondary to the massive microvessel damage, leading to ischemia induced by hemorrhagic SVMPs. Since P-III SVMPs are readily immunodepleted by polyvalent antivenom, this explains the high efficacy of this antivenom to neutralize the hemorrhagic, myotoxic and lethal effects of A. picadoi venom. In contrast, in the case of A. mexicanus venom, neutralization of hemorrhagic activity is highly efficient whereas neutralization of lethality requires a higher volume of antivenom. Since P-III SVMPs comprise only 3.4 % of the venom of A. mexicanus (Angulo et al, 2008), it is suggested that the lethality of this venom by the intraperitoneal route is probably due to the action of PLA2s. This is in line with the relatively low toxicity of the venom, i.e., high LD50, since myotoxic PLA2s from Central American viperid venoms are lethal only at relatively high doses (Gutiérrez and Lomonte, 1995; Gutiérrez and Lomonte, 1997). Hence, the different proportions of P-III SVMPs and PLA2s in these venoms largely explain the variations in the neutralization of lethality. On the other hand, the neutralization of the proteolytic activity on azocasein by the Costa Rican polyvalent antivenom is 31 and 12 times less effective than the neutralization of the hemorrhagic activity of the venoms of A. picadoi and A. mexicanus, respectively, despite the fact that both effects are due to SVMPs, as judged by the ability of EDTA to abolish them. Such apparent discrepancy is likely due to the fact that the hemorrhagic activity is largely due to P-III SVMPs whereas the proteolytic activity is mainly dependent on the action of P-I SVMPs, which in general have a higher enzymatic activity, albeit being less toxic, than P-III SVMPs (Gutiérrez et al, 2009b). Since P-I SVMPs are immunodepleted to a lesser extent than P-III SVMPs by the antivenom, antivenomic and neutralization results clearly match regarding the neutralization of hemorrhagic and proteolytic effects.
CONCLUSIONS
Our observations demonstrate an extensive cross-neutralization of A. mexicanus and A. picadoi venoms by the polyvalent antivenom used for the treatment of viperid envenomings in Central America.
Antivenomic observations evidenced that antivenoms readily immunodeplete P-III SVMPs from both venoms, but immunodepletion of PLA2s, CRISPs and some P-I SVMPs and serine proteinases was achieved only to a partial extent, thus revealing the scarcity of (high-affinity) antibodies against these proteins.
These findings, together with previous antivenomic studies, stress the need to search for immunization strategies aimed at enhancing the immune response against these poorly immunogenic venom toxins in order to increase the efficacy and neutralizing potency of antivenoms.
Acknowledgments
This study was supported by projects from the Vicerrectoría de Investigación, Universidad de Costa Rica (741-A7-611), CRUSA-CSIC (2007CR0004), CONARE, and grants BFU2007-61563 and BFU2010-17373 from the Ministerio de Ciencia e Innovación, Madrid, Spain.
STATEMENT OF COMPETING INTERESTS
None declared.
REFERENCES
- Alape-Girón A, Sanz L, Escolano J et al. Snake venomics of the lancehead pitviper Bothrops asper: geographic, individual and ontogenetic variations. J Proteome Res. 2008;7:3556–3571. doi: 10.1021/pr800332p. [DOI] [PubMed] [Google Scholar]
- Angulo Y, Estrada R, Gutiérrez JM. Clinical and laboratory alterations in horses during immunization with snake venoms for the production of polyvalent (Crotalinae) antivenom. Toxicon. 1997;35:81–90. doi: 10.1016/s0041-0101(96)00077-3. [DOI] [PubMed] [Google Scholar]
- Angulo Y, Escolano J, Lomonte B, Gutiérrez JM, Sanz L, Calvete JJ. Snake venomics of Central American pitvipers. Clues for rationalizing the distinct envenomation profiles of Atropoides nummifer and Atropoides picadoi. J Proteome Res. 2008;7:708–719. doi: 10.1021/pr700610z. [DOI] [PubMed] [Google Scholar]
- Bogarín G, Morais JF, Yamaguchi IK et al. Neutralization of crotaline snake venoms form Central and South America by antivenoms produced in Brazil and Costa Rica. Toxicon. 2000;38:1429–1441. doi: 10.1016/s0041-0101(99)00236-6. [DOI] [PubMed] [Google Scholar]
- Bolaños R. Nuevos Recursos contra el Ofidismo en Centroamérica. Universidad de Costa Rica; San José, Costa Rica: 1971. [Google Scholar]
- Bolaños R. Toxicity of Costa Rican snake venoms for the white mouse. Am J Trop Med Hyg. 1972;21:60–63. doi: 10.4269/ajtmh.1972.21.360. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Sanz L, Angulo Y, Lomonte B, Gutiérrez JM. Venoms, venomics, antivenomics. FEBS Lett. 2009a;583:1736–1743. doi: 10.1016/j.febslet.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Borges A, Segura A et al. Snake venomics and antivenomics of Bothrops colombiensis, a medically important pitviper of the Bothrops atrox-asper complex endemic to Venezuela: Contributing to its taxonomy and snakebite management. J Proteomics. 2009b;72:227–240. doi: 10.1016/j.jprot.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Cid P, Sanz L et al. Antivenomic assessment of the immunological reactivity of EchiTAb-Plus-ICP®, an antivenom for the treatment of snakebite envenoming in sub-Saharan Africa. Am J Trop Med Hyg. 2010a;82:1194–1201. doi: 10.4269/ajtmh.2010.09-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ, Sanz L, Cid P et al. Snake venomics of the Central American rattlesnake Crotalus simus and the South American Crotalus durissus complex points to neurotoxicity as an adaptive paedomorphic trend along Crotalus dispersal in South America. J Proteome Res. 2010b;9:528–544. doi: 10.1021/pr9008749. [DOI] [PubMed] [Google Scholar]
- Calvete JJ. Antivenomics and venom phenotyping: a marriage of convenience to address the performance and range of clinical use of antivenoms. Toxicon. 2010 doi: 10.1016/j.toxicon.2009.12.015. in press. [DOI] [PubMed] [Google Scholar]
- Campbell JA, Lamar WW. The Venomous Reptiles of the Western Hemisphere. Cornell University Press, Ithaca; United States of America: 2004. [Google Scholar]
- Chippaux JP, Williams V, White J. Snake venom variability: methods of study, results and interpretation. Toxicon. 1991;29:1279–1303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- Correa-Netto C, Teixeira-Araujo R, Aguiar AS et al. Immunome and venome of Bothrops jararacussu: a proteomic approach to study molecular immunology of snake toxins. Toxicon. 2010;55:1222–1235. doi: 10.1016/j.toxicon.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Dole VP. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956;35:150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasman DG, editor. Practical Handbook of Biochemistry and Molecular Biology. CRC, Press Boston; United States of America: 1992. (editor) [Google Scholar]
- Gené JA, Roy A, Rojas G, Gutiérrez JM, Cerdas L. Comparative study on the coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon. 1989;27:841–848. doi: 10.1016/0041-0101(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Gené JA, Rojas G, Cerdas L. Neutralization of proteolytic and hemorrhagic activities of Costa Rican snake venoms by a polyvalent antivenom. Toxicon. 1985;23:887–893. doi: 10.1016/0041-0101(85)90380-0. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Rojas G, Lomonte B, Gené JA, Cerdas L. Comparative study of the edema-forming activity of Costa Rican snake venoms and its neutralization by a polyvalent antivenom. Comp Biochem Physiol C. 1986;85:171–175. doi: 10.1016/0742-8413(86)90069-1. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Lomonte B, Chaves F, Moreno E, Cerdas L. Pharmacological activities of a toxic phospholipase A isolated from the venom of the snake Bothrops asper. Comp Biochem Physiol C. 1986;84:159–164. doi: 10.1016/0742-8413(86)90183-0. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Rojas G, Lomonte B et al. Standardization of assays for testing the neutralizing ability of antivenoms. Toxicon. 1990;28:1127–1129. doi: 10.1016/0041-0101(90)90110-s. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Rojas G, Bogarín G, Lomonte B. Evaluation of the neutralizing ability of antivenoms for the treatment of snake bite envenoming in Central America. In: Bon C, Goyffon M, editors. Envenomings and Their Treatments. Fondation Marcel Mérieux; Lyon, France: 1996. pp. 223–231. (Eds) [Google Scholar]
- Gutiérrez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33:1405–1424. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. In: Kini RM, editor. Venom Phospholipase A2 Enzymes. Structure, Function and Mechanism. Wiley; Chichester, United Kingdom: 1997. pp. 321–352. (ed) [Google Scholar]
- Gutiérrez JM, Rucavado A, Escalante T, Díaz C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon. 2005;45:997–1011. doi: 10.1016/j.toxicon.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Sanz L, Escolano J et al. Snake venomics of the Lesser Antillean pit vipers Bothrops caribbaeus and Bothrops lanceolatus: correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J Proteome Res. 2008;7:4396–4408. doi: 10.1021/pr8003826. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Lomonte B, León G et al. Snake venomics and antivenomics: proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J Proteomics. 2009a;72:165–182. doi: 10.1016/j.jprot.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Rucavado A, Escalante T. Snake venom metalloproteinases. Biological roles and participation in the pathophysiology of envenomation. In: Mackessy SP, editor. Handbook of Venoms and Toxins of Reptiles. CRC Press, Boca Raton; United States of America: 2009b. pp. 115–138. (Ed) [Google Scholar]
- Gutiérrez JM. Snakebite envenomation in Central America. In: Mackessy S.P., editor. Handbook of Venoms and Toxins of Reptiles. CRC Press, Boca Raton; United States of America: 2009. pp. 491–507. [Google Scholar]
- Gutiérrez JM, Sanz L, Flores-Díaz M et al. Impact of regional variation in Bothrops asper snake venom on the design of antivenoms: integrating antivenomics and neutralization approaches. J Proteome Res. 2010;9:564–577. doi: 10.1021/pr9009518. [DOI] [PubMed] [Google Scholar]
- Instituto Clodomiro Picado. Determinación de Actividades Tóxicas de Venenos de Serpientes y su Neutralización por Antivenenos. Manual de Métodos de Laboratorio. Instituto Clodomiro Picado; San José, Costa Rica: 2008. [Google Scholar]
- Lomonte B, Escolano J, Fernández J et al. Snake Venomics and Antivenomics of the Arboreal Neotropical Pitvipers Bothriechis lateralis and Bothriechis schlegelii. J Proteome Res. 2008;7:2445–57. doi: 10.1021/pr8000139. [DOI] [PubMed] [Google Scholar]
- Padlan EA, Silverton EW, Sheriff S, Cohen G, Smith-Gill SJ, Davies DR. Structure of an antibody-antigen complex: crystal structure of the HyHEL-10 Fab-lysozyme complex. Proc Natl Acad Sci USA. 1989;86:5938–5942. doi: 10.1073/pnas.86.15.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas G, Jiménez JM, Gutiérrez JM. Caprylic acid fractionation of hyperimmune horse plasma: description of a simple procedure for antivenom production. Toxicon. 1994;32:59–67. doi: 10.1016/0041-0101(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Rojas G, Gutiérrez JM, Gené JA, Gómez M, Cerdas L. Neutralización de las actividades tóxicas y enzimáticas de cuatro venenos de serpientes de Guatemala y Honduras por el antiveneno polivalente producido en Costa Rica. Rev Biol Trop. 1987;35:59–67. [PubMed] [Google Scholar]
- Rojas E, Saravia P, Angulo Y et al. Venom of the crotaline snake Atropoides nummifer (jumping viper) from Guatemala and Honduras: comparative toxicological characterization, isolation of a myotoxic phospholipase A2 homologue and neutralization by to antivenoms. Comp Biochem Physiol C. 2001;129:151–162. doi: 10.1016/s1532-0456(01)00198-3. [DOI] [PubMed] [Google Scholar]
- Saravia P, Rojas E, Escalante T et al. The venom of Bothrops asper from Guatemala: toxic activities and neutralization by antivenoms. Toxicon. 2001;39:401–405. doi: 10.1016/s0041-0101(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Saravia P, Rojas E, Arce V et al. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: pathophysiological and therapeutic implications. Rev Biol Trop. 2002;50:337–346. [PubMed] [Google Scholar]
- Segura A, Castillo MC, Núñez V et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon. 2010 doi: 10.1016/j.toxicon.2010.07.001. in press. [DOI] [PubMed] [Google Scholar]
- Theakston RDG, Reid HA. Development of simple standard assay procedures for the characterization of snake venoms. Bull World Health Org. 1983;61:949–956. [PMC free article] [PubMed] [Google Scholar]
- Theakston RDG. Characterization of venoms and standardization of antivenoms. In: Harris JB, editor. Natural Toxins. Animal, Plant and Microbial. Clarendon Press; Oxford, United Kingdom: 1986. pp. 287–303. (Ed) [Google Scholar]
- Theakston RDG, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541–557. doi: 10.1016/s0041-0101(02)00393-8. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente C, Moreno E, Sittenfeld A, Lomonte B, Gutiérrez JM. An electrophoretic study on phospholipase A2 isoenzymes in the venoms of Central American crotaline snakes. Toxicon. 1992;30:815–823. doi: 10.1016/0041-0101(92)90379-j. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Shih CH, Huang TF. A novel P-I class metalloproteinase with broad substrate-cleaving activity, agkislysin, from Agkistrodon acutus venom. Biochem Biophys Res Comm. 2004;324:224–230. doi: 10.1016/j.bbrc.2004.09.031. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Progress in the Characterization of Venoms and Standardization of Antivenoms. World Health Organization; Geneva, Switzerland: 1981. WHO Offset Publication No. 58. [Google Scholar]
- World Health Organization. Guidelines for the Production, Control and Regulation of Antivenom Immunoglobulins. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]