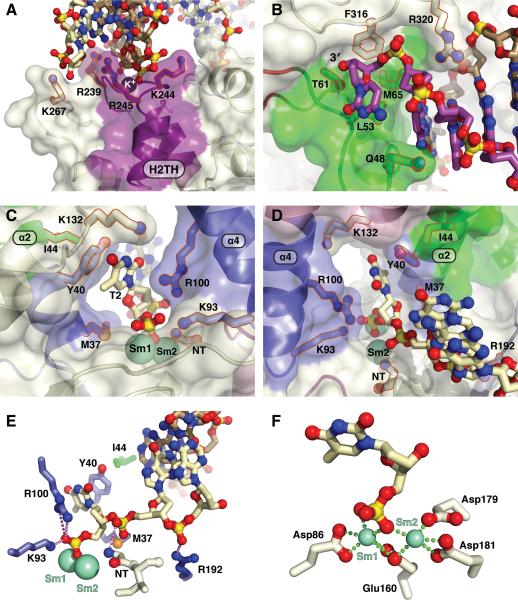
Figure 3. Key FEN1 Structural Elements in the FEN:Sm3+:Product Complex.
(A) The H2TH:K+ motif and surrounding basic residues (purple) forms an electrostatic track for the downstream dsDNA minor groove.
(B) The 3′-flap binding pocket (green) binds the 3′-hydroxyl and the unpaired nt sugar moiety.
(C) Rear view of the active site, opposite the template DNA interacting region, shows the helical gateway (blue), active site residues and product DNA. The cleaved 5′-nt sits in the helical gateway formed by α2 and α4 and under the cap. Figures S2A-C show C-capping in the gateway.
(D) Front view of the active site shows the gateway and 5′-flap strand approach to the active site.
(E) FEN1 binds to the -1 to -3 phosphates of the 5′ -flap product strand.
(F) Four conserved FEN1 carboxylate residues and two phosphate oxygens of the +1 5′-phosphate in the DNA directly coordinate the two Sm3+ ions. Distances are 2.3 – 2.5 Å. Figure S2D shows all seven highly conserved carboxylates and bound waters.