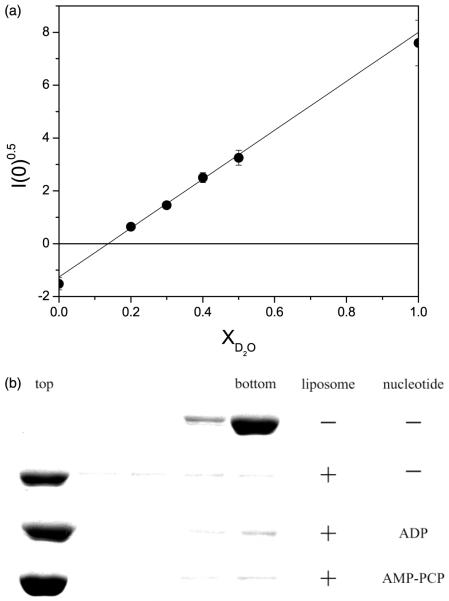
Figure 1.
(a) Contrast variation experiments to determine the match point of E. coli SUV. To make SUV, 0.4 ml of 25 mg/ml of chloroform-dissolved E. coli polar lipid extract (Avanti Polar Lipids, Inc., AL) was transferred to a 12 ml Pyrex tube. A thin film of lipid was formed under a stream of filtered nitrogen and residual chloroform was removed by high vacuum overnight. The lipid film was then re-hydrated in 1 mM DTT, 50 mM potassium phosphate (pH 7.5), 50 mM KCl, 1 mM EDTA. SUV were formed by sonicating until the solution was optically clear. The contrast variation experiments required different volume fractions of 2H2O to determine the lipid contrast match point at which the scattering intensity from the SUV is zero. For contrast variation experiments, the lipid was resuspended separately in H2O and 2H2O buffer to make the two SUV solutions. The H2O and 2H2O SUV solutions were mixed in different proportions to produce six SUV solutions of different 2H2O volume fractions. The lipid concentration used for contrast variation experiments was 10 mg/ml. The square-root of scattering intensity I(0)0.5, from the SUV as a function of 2H2O volume fraction is shown. The contrast match point can be determined from this plot to be X2H2O = 0.143. At a 2H2O volume fraction of 0.143, the scattering intensity from the lipid vesicles is zero, indicating the scattering density contrast between the SUV and the buffer solution is zero. Further experiments on SecA/SUV solutions were therefore conducted at this 2H2O concentration to eliminate the scattering from the SUV. (b) Association of SecA with SUV analyzed by flotation gradient centrifugation. SecA (1.5 mg/ml) was mixed with E. coli SUV (12 mg/ml) in the aforementioned phosphate buffer with density of 1.29 g/ml adjusted with metrizamide. Magnesium acetate (60 μM) was added when ADP (60 μM) or AMP-PNP (2 mM) was included in buffer. Samples (200 μl) were layered below 400 μl of corresponding buffers with density of 1.27 g/ml. Samples were centrifuged (for 33 hours, at 4°C, at 35,000 rpm) in a Beckman SW50.1 rotor with adapters for 5 × 41 mm Beckman Ultraclear centrifuge tubes, six 100 μl fractions were harvested and the top three and bottom two fractions analyzed by SDS-PAGE and staining with Coomassie brilliant blue.