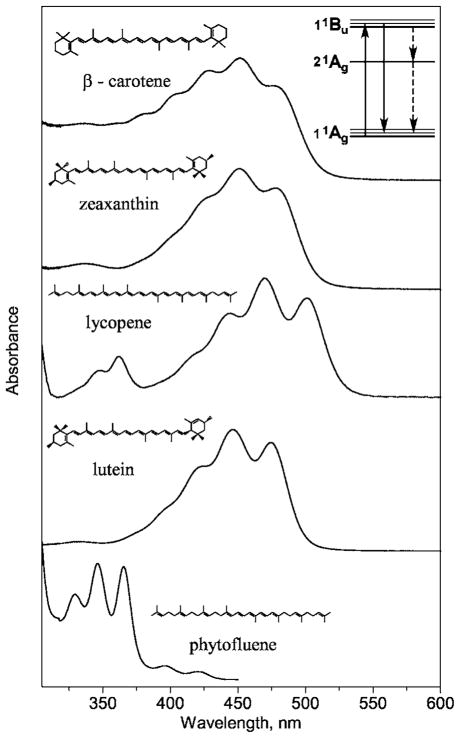
Fig. 1.
Absorption spectra, molecular structures, and energy level scheme of major carotenoid species found in human tissue, including β-carotene, zeaxanthin, lycopene, lutein, and phytofluene. Carotenoid molecules, which feature an unusual even parity excited state (see inset). As a consequence, absorption transitions are electricdipole allowed in these molecules but spontaneous emission is forbidden. The resulting absence of any strong fluorescence in carotenoids is the main reason for the possibility to use RRS, shown as a solid, downward-pointing arrow (optical transition) in the inset, as a noninvasive means of carotenoid detection in human tissue. All carotenoid molecules feature a linear, chainlike conjugated carbon backbone consisting of alternating carbon single (C—C) and double bonds (C==C) with varying numbers of conjugated C==C double bonds, and a varying number of attached methyl side groups. Beta-carotene, lutein, and zeaxanthin feature additional ionone rings as end groups. In beta-carotene and zeaxanthin, the double bonds of these ionone rings add to the effective C==C double bond length of the molecules. Lutein and zeaxanthin have an OH group attached to the ring. Lycopene has 11 conjugated C==C bonds, beta carotene has 11, zeaxanthin has 11, lutein has 10, and phytofluene has 5. The absorptions of all species occur in broad bands in the blue/green spectral range, with the exception of phytofluene, which as a consequence of the shorter C==C conjugation length absorbs in the near UV. Note also, that a small (~10 nm) spectral shift exists between lycopene and lutein absorptions. The spectral shifts can be explored with RRS to selectively detect some of the carotenoids existing as a mixture in human tissues.