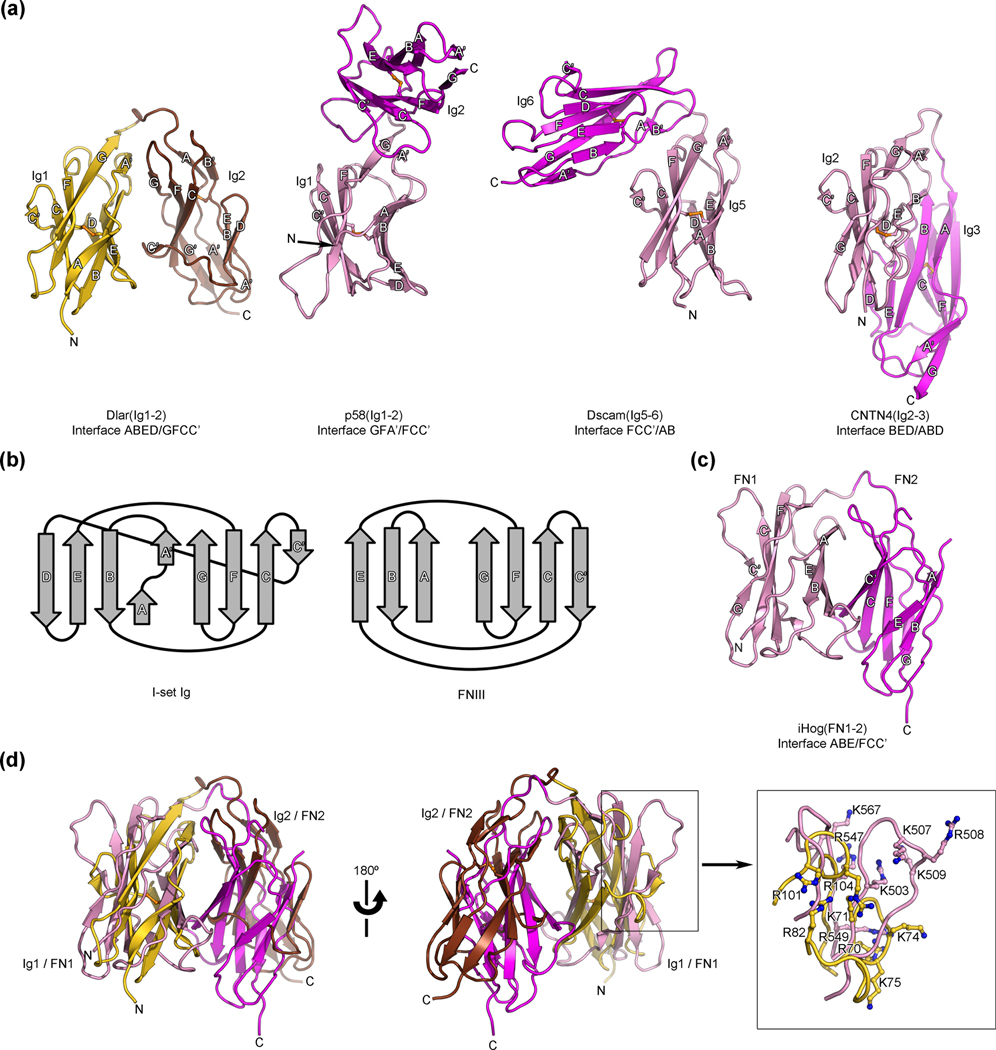
Fig. 3.
Structural comparisons of the antiparallel arrangement of Ig domains 1 and 2 of Dlar with other horseshoe-like structures. (a) Comparison of the Dlar(Ig1-2) structure (left, colored gold and brown) with three structures of tandem Ig repeats that adopt a horseshoe-like conformation. The distinct conformations of horseshoe-like arrangements of these tandem β-sandwiches repeats arise from interactions between residues found in distinct strands, which are listed below each structure. In each of the compared structures, the N-terminal and C-terminal Ig repeats are colored pink and magenta, respectively. Disulfide bonds are shown as orange ball-and-stick models. The letters N and C indicate the N- and C-termini, respectively. These views of Dlar(Ig1-2), p58(Ig1-2), CNTN4(Ig2-3) and Dscam(Ig5-6) were obtained by superimposing their N-terminal Ig repeats in an effort to highlight the distinct positions of the C-terminal Ig repeats that result from changes in the contacting faces in each of the horseshoe-like structures. (b) Topology diagrams for I set Ig domains and FNIII domains highlighting the similarities between the two folds 45. (c) Ribbon diagram depicting the horseshoe-like arrangement observed for the tandem FNIII repeats of Drosophila iHog. The first and second FNIII repeat of iHog are colored pink and magenta, respectively. This view was obtained by superimposing the 1st FNIII iHog and the 1st Ig repeat of Dlar using secondary structure matching. (d) Overlay of the Ig1–Ig2 fragment of Dlar with the first (pink) and second (magenta) FNIII domains of Drosophila iHog. The HSPG-binding site for iHog and the presumed glycosaminoglycan-binding site for Dlar partially overlap and are shown in the boxed view.