Table 2.
ATRA Using Photoredox Catalysisa
| entry | substrate | olefin | product | yieldb |
|---|---|---|---|---|

|
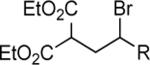
|
|||
| 1 | 4 | R = CH2NHTs | 67c | |
| 2 | 4 | R = CH2NHBoc | 99 | |
| 3 | 4 | R = (CH2)4OH | 95 | |
| 4 | 4 | R = (CH2)3OH | 99 | |
| 5 | 4 | R = (CH2)4OTBS | 90 | |
| 6 | 4 | R = (CH2)4OCH2Ph | 92 | |
| 7 | 4 | R = (CH2)3Br | 92 | |
| 8 | 4 | R = (CH2)4CO2Et | 99 | |
| 9 | 4 |
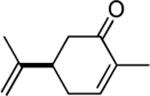
|
|
95d |
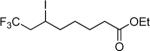
| 10 | CF3I |
|
|
90e,f |
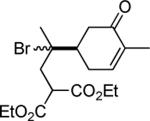
| 11 | CF3I |
|
|
81e,f |
| 12 |
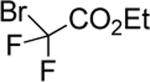
|
|
|
93 |
| 13 |
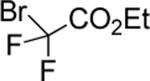
|
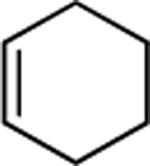
|
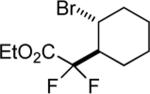
|
75 |
| 14 | CCl3Br |
|
|
87f |
| 15 |
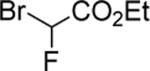
|
|
|
99d |
| 16 |
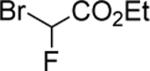
|
|
|
84d |
Reaction conditions: 1 (1.0 mol %), haloalkane (2.0 equiv), and LiBr (2.0 equiv) in DMF/H2O (1:4).
Isolated yield (%) after purification on SiO2.
90% brsm.
dr 1:1.
Excess of haloalkane was used.
No LiBr added.
