Figure 5.
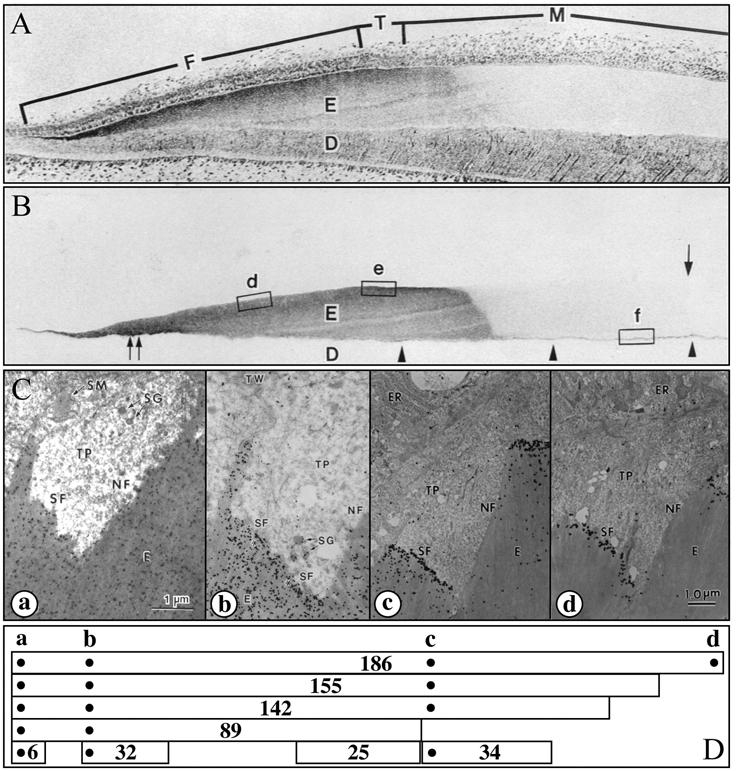
Enamelin localization in developing enamel. (A,B) Consecutive sections of a developing porcine incisor. The top section is stained with toluidine blue; the second section is immunostained with the 32-kDa enamelin antibody (Uchida et al., 1991a). The 32-kDa enamelin signal is observed throughout the secretory stage in the enamel matrix, from the DEJ to the surface. However, because enamelin is extensively processed by proteases and the C-terminal parts are removed from the matrix, its distribution depends upon which part of the protein is recognized by the antibody. (C,D) Transmission electron microscopy (TEM) showing immunogold staining patterns of forming enamel near the ameloblast Tomes’ processes using affinity-purified anti-peptide antibodies raised against the enamelin (a) N-terminus (Dohi et al., 1998), (b) 32-kDa cleavage product (Uchida et al., 1991a), (c) 34-kDa cleavage product (Hu et al., 1997a), and (d) C-terminus (Hu et al., 1997a). Note that each antibody shows a different localization pattern. Below the TEMs are diagrams showing enamelin (186 kDa) and known cleavage products (155, 142, 89, 34, 32, 25, and 6 kDa) and the positions of the sequences used to make antibodies (dots) (Hu and Yamakoshi, 2003). An important observation is that the enamelin C-terminus is found only at the mineralization front (d).
