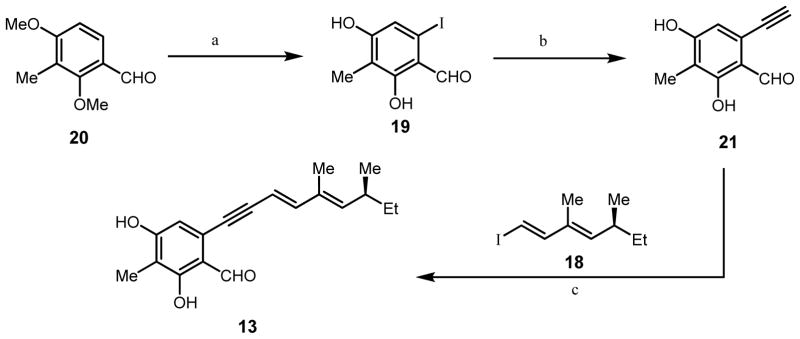
Scheme 2.
Synthesis of alkynylbenzaldehyde 13.
a) N,N,N′-trimethylethylenediamine, n-BuLi, THF, 0°C 30 min.; n-BuLi, THF, −20°C, 16 h, then 1,2-diiodoethane; BBr3, CH2Cl2, 18 h (33% yield three steps b) CuI, trimethylsilylacetylene, (t-Bu)3P-HBF4, PdCl2(CH3CN)4, diisopropylamine, rt, 12 h, then K2CO3, MeOH, rt, 1 h 80%, two steps; c) Pd(PPh3)4, CuI, Et3N, 12 h, rt, 55%.