Table 1.
Oxidative dearomatization employing sparteine surrogates.a
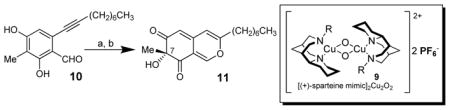 | |||
|---|---|---|---|
| entry | ligand | product | yieldb (ee) |
| 1 |
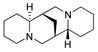 4 |
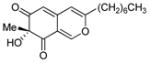 (R)-11 |
84% (98%)2 |
| 2 |
5 |
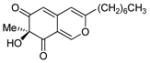 (S)-11 |
63% (92%) |
| 3 |
6 |
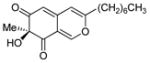 (S)-11 |
70% (95%) |
| 4 |
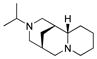 7 |
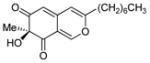 (S)-11 |
54% (74%) |
| 5 |
8 |
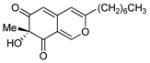 (R)-11 |
33% (11%) |
Conditions: (a) 2.2 equiv. Cu(CH3CN)4PF6, 2.4 equiv. ligand, 1.6 equiv. DIEA, 2.4 equiv. DMAP, O2, −78°C to −10°C; (b) aq. H2PO4/K2HPO4 buffer (pH 7.2), CH3CN, RT [b] Isolated yield after silica gel chromatography. DIEA = N,N-diisopropylethylamine, DMAP = 4-(dimethylamino)pyridine.
