Abstract
Idiopathic pulmonary arterial hypertension (IPAH) is a disease of unknown cause that is characterized by elevated pulmonary arterial pressure and pulmonary vascular resistance attributable to vasoconstriction and vascular remodeling of small pulmonary arteries. Vascular remodeling includes hypertrophy and hyperplasia of smooth muscle (medial hypertrophy) accompanied in up to 80% of the cases by the formation of occlusive plexiform lesions (plexogenic arteriopathy). Patients tend to be unresponsive to vasodilator therapy and have a poor prognosis for survival when plexogenic arteriopathy progressively obstructs their pulmonary arteries. Research is needed to understand and treat plexogenic arteriopathy, but advances have been hindered by the absence of spontaneously developing lesions in existing laboratory animal models. Young domestic fowl bred for meat production (broiler chickens, broilers) spontaneously develop IPAH accompanied by semi-occlusive endothelial proliferation that progresses into fully developed plexiform lesions. Plexiform lesions develop in both females and male broilers, and lesion incidences (lung sections with lesions/lung sections examined) averaged approximately 40% in 8 to 52 week old birds. Plexiform lesions formed distal to branch points in muscular interparabronchial pulmonary arteries, and were associated with perivascular mononuclear cell infiltrates. Serotonin (5-hydroxytryptamine, 5-HT) is a potent vasoconstrictor and mitogen known to stimulate vascular endothelial and smooth muscle cell proliferation. Serotonin has been directly linked to the pathogenesis of IPAH in humans, including IPAH linked to serotonergic anorexigens that trigger the formation of plexiform lesions indistinguishable from those observed in primary IPAH triggered by other causes. Serotonin also plays a major role in the susceptibility of broilers to IPAH. This avian model of spontaneous IPAH constitutes a new animal model for biomedical research focused on the pathogenesis of IPAH and plexogenic arteriopathy.
Key Terms: animal model; domestic fowl (chickens, Gallus gallus domesticus); lungs; microparticle injection method; pathology; plexiform lesions; pulmonary hypertension; serotonin
4.1 Idiopathic Pulmonary Arterial Hypertension (IPAH) in Humans
IPAH, formerly known as primary pulmonary hypertension (PPH), is a disease of unknown cause characterized by elevated pulmonary vascular resistance (PVR) and a mean pulmonary arterial pressure (PAP) ≥ 25 mm Hg at rest in the absence of other chronic lung or heart disease. The increased PVR seen in IPAH has been attributed to vasoconstriction and remodeling of the pulmonary vasculature, diminished vascular compliance, and vascular occlusion by thrombi. The pathogenesis of IPAH is not clearly understood, and the likelihood exists that IPAH encompasses a variety of etiologies exhibiting common end stage lung pathology. Patients with IPAH are partitioned into ‘familial’ and ‘sporadic’ categories. Assignment to the ‘familial’ category is based on genealogical evidence and confirmation of the presence of a key mutation in the bone morphogenetic protein receptor type II (BMPR2) gene. Familial PAH (FPAH) encompasses approximately 12% of all PAH patients, although as many as 25% of the patients assigned to the sporadic IPAH category also test positive for mutation of the BMPR2 gene (Wagenvoort and Wagenvoort, 1970; Loyd and Newman, 1997; Pietra, 1997; Rubin, 1997; Voelkel et al., 1997; Lee et al., 1998; Nauser and Stites, 2001; McLaughlin, 2002; Newman et al., 2004; Simonneau et al., 2004; Morse, 2005; Stenmark et al., 2009).
4.2 Pulmonary Vascular Pathology in IPAH
The early pathogenesis of IPAH is relatively poorly understood because most patients seek medical evaluation only after their disease has progressed sufficiently to provoke overt clinical symptoms. Pathological evaluations traditionally indicated that early pulmonary vascular remodeling progressively evolves over a number of years into the severe obstructive vascular pathology observed in patients confirmed to have advanced IPAH (Wagenvoort and Wagenvoort, 1970; Voelkel et al., 1997). In very young patients increases in PVR and PAP appear to be correlated with vasoconstriction and medial hypertrophy of small (<400 µ diameter) pre-acinar to intra-acinar pulmonary arteries. Medial hypertrophy encompasses hypertrophy and hyperplasia of the smooth muscle fibers in the tunica media of muscular arteries, distal extension (neomuscularization) of smooth muscle into non-muscularized intra-acinar arteries, and increased densities of elastin and collagen fibers within the tunica media (Wagenvoort and Wagenvoort, 1970; Pietra, 1997; Voelkel et al., 1997; Yi et al., 2000; Pietra et al., 2004). Medial hypertrophy often is accompanied in smaller arterioles by intimal thickening attributable to the accumulation of one or more layers of myofibroblasts and fibrous matrix proteins within the neointimal space between the endothelium (tunica intima) and the internal elastic lamina (Palevsky et al., 1989; Yi et al., 2000; Pietra et al., 2004). In more severe or advanced stages, small pre- and intra-acinar arterioles typically exhibit complex lesions that functionally occlude the vessel’s lumen, including concentric laminar intimal proliferation (”onionskin” or concentric-obliterative lesions), and glomeruloid-like plexiform lesions. Plexiform lesions traditionally have been regarded as characteristic of sustained, rapidly progressing or severe vasoconstrictive pulmonary hypertension, and are present in the lungs of up to 80% of the patients with severe IPAH. Survival times are reported to be shortest when PAH patients’ biopsies reveal the presence of plexiform lesions (Wagenvoort and Wagenvoort, 1970; Palevsky et al., 1989; Tuder et al., 1994; Voelkel et al., 1997; Pietra et al., 2004). Plexiform lesions have been described in several diseases that evoke PAH and are not considered pathognomonic for the idiopathic or familial PAH categories (Voelkel et al., 1997; Pietra, 1997; Yi et al., 2000; Pietra et al., 2004).
Plexiform lesions form immediately downstream from branching points in small muscular pre- and intra-acinar arterioles. Arteriole bifurcation sites are thought to promote the development of complex lesions as a consequence of localized turbulent blood flow and shear stress that damage the normally quiescent endothelium (Mecham et al., 1987; Frid et al., 1997; Kelly et al., 1998; Stevens et al., 2001; Aird, 2003; Archer et al., 2004; Cool et al., 2005). The resulting apoptosis of normal endothelial cells is thought to permit clones of apoptosis-resistant endothelial cells to proliferate, leading to the progressive formation of plexiform lesions (Tuder et al., 1994; Lee et al., 1998; Botney, 1999; Taraseviciene-Stewart et al., 2001; Budhiraja et al., 2004; Humbert et al., 2004; Cool et al., 2005; Sakao et al., 2005). Plexiform lesions have been described as dynamic angiogenic lesions driven by disordered or neoplastic-like endothelial proliferation and myofibroblast infiltration (Lee et al., 1998; Cool et al., 1999; Yi et al., 2000; Berger et al., 2001; Tuder et al., 2001). Endothelial cells in most but not all of the plexiform lesions from patients with IPAH tend to be monoclonal rather than polyclonal in origin, supporting the concept that somatic gene mutations promote proliferation of the altered cells. Endothelial cells from plexiform lesions in anorexigen-triggered pulmonary arterial hypertension (PAH) also tend to be monoclonal in origin, whereas those from patients with secondary PAH are polyclonal in origin (Lee et al., 1998; Tuder et al., 1998b). Dysregulated endothelial cells proliferate until the lumen of an arteriole is functionally obstructed. Slit-like anastomosing endothelial channels supported by connective tissue and myofibroblasts canalize the plexiform obstruction. Newly formed endothelial channels circumvent the obstruction by penetrating through the arterial wall into the perivascular connective tissue (Tuder et al., 1994; Cool et al., 1997, 1999; Pietra, 1997; Pietra et al., 2002; Stevens, 2005). Thrombi and platelet aggregates commonly are found within established plexiform lesions, an anticipated response to endothelial changes that create thrombogenic surfaces. In the absence of anticoagulant therapy, ongoing in situ thrombotic occlusion further complicates the vascular pathology and increases the PVR (Heath and Edwards, 1958; Voelkel et al., 1997; Farber and Loscalzo, 1999; Archer and Rich, 2000; Yi et al., 2000; Humbert et al., 2004; Hoeper et al., 2006). Inflammatory cells tend to infiltrate the perivascular zone of plexiform lesions, and fibrosis can develop in the intimal and adventitial layers of the lesion (Tuder et al., 1994; Voelkel et al., 1997; Dorfmüller et al., 2003; Nicolls et al., 2005).
Endothelial cells within plexiform lesions over-express several key markers of angiogenesis, including vascular endothelial growth factor (VEGF) and its receptor (VEGFR-2). At the central core of plexiform lesions the endothelial cells are actively proliferating and do not express transforming growth factor-β (TGF-β) signaling molecules, whereas at the periphery of the plexiform lesion the endothelial cells appear to be involved in de novo angiogenesis and actively express TGF-β signaling molecules (Cool et al., 1999; Geiger et al., 2000; Tuder et al., 2001; Richter et al., 2004; Abe et al., 2010). Endothelial cells within plexiform lesions also tend to over-express components of pathways leading to endothelium-derived vasoconstrictor production (e.g., leukotrienes, thromboxane, and endothelin) (Giaid et al., 1993; Wright et al., 1998; Budhiraja et al., 2004), and variably express components of pathways leading to endothelium-derived vasodilator production (e.g., nitric oxide and prostacyclin) (Giaid and Saleh, 1995; Mason et al., 1998; Tuder et al., 1999; Berger et al., 2001). Conflicting observations of both enhanced (Mason et al., 1998; Berger et al., 2001) and suppressed (Giaid and Saleh, 1995) nitric oxide synthase (NOS) expression within plexiform lesions may reflect variable leukocyte infiltration as well as the distribution of affected arterioles within a transitional zone between macro-vascular (high NOS expressing) and micro-vascular (low NOS expressing) endothelial cell phenotypes (Stevens et al., 2001; Stevens, 2005). Nitric oxide (NO) inhibits platelet aggregation, reduces pulmonary artery contractile responses to serotonin (5-hydroxytryptamine, 5-HT) and endothelin-1 (ET-1), and modulates (attenuates) mitogenic responses to ET-1 and 5-HT (Martinez-Lemus et al., 1999; Tyler et al., 1999; Villamor et al., 2002; Jones et al., 2003; Wideman et al., 2004, 2007).
The precise stimuli responsible for initiating vasoconstriction, medial hypertrophy, and plexiform lesion formation remain unknown. The sustained vasoconstriction and medial hypertrophy of small pulmonary arterioles may reflect enhanced production of or sensitivity to endothelium- or leukocyte-derived pro-mitogenic vasoconstrictors (5-HT, ET-1), attenuated production of or sensitivity to endothelium-derived anti-mitogenic vasodilators (NO, PGI2), altered function of K+ ion channels, and epigenetically triggered apoptosis followed by proliferation of apoptosis-resistant smooth muscle and endothelial cells (Giaid et al., 1993; Giaid and Saleh, 1995; Voelkel et al., 1997; Wright et al., 1998, Tuder et al., 1999; Archer and Rich, 2000; Eddahibi et al., 2001a, 2002; Taraseviciene-Stewart et al., 2001; Archer et al., 2004; Budhiraja et al., 2004; Humbert et al., 2004; Cogolludo et al., 2006). Current concepts suggest the pathology of pulmonary hypertension can be divided into vessel wall abnormalities attributable primarily to vasoconstriction (e.g., medial hypertrophy and adventitial thickening) and concurrently or independently evolving endothelial damage and clonal proliferation attributable to elevated shear stress (e.g., concentric intimal thickening and plexiform lesion formation) (Tuder et al., 2001; Richter et al., 2004; Cool et al., 2005; Stevens, 2005; Stenmark et al., 2009). To the extent that medial hypertrophy and plexiform lesion formation are independent rather than sequential processes, then pharmacologic therapies designed to reverse smooth muscle vasoconstriction are unlikely to impede the deteriorating pathology attributable to progressive plexogenic pulmonary arteriopathy. Increases in PVR and PAP appear to be most amenable to pharmacologic reversal through vasodilator administration or vasoconstrictor receptor blockade when the vascular pathology is limited primarily to medial hypertrophy. Patients confirmed by cardiac catheterization to have severe PAH are more likely to exhibit complex obstructive pulmonary vascular pathology and are less likely to benefit from pharmacologic interventions designed to reduce their PVR (Wagenvoort and Wagenvoort, 1970; Barst, 1986; Palevsky et al., 1989; Voelkel et al., 1997). New approaches are needed to understand and treat advanced cases of PAH involving plexogenic arteriopathy (Nishimura et al., 2002, 2003; Taraseviciene-Stewart et al., 2006; Stenmark et al., 2009; Abe et al., 2010; Firth et al., 2010).
4.3 Pulmonary Vascular Pathology in Mammalian Models of PAH
Attempts to understand the pathogenesis of complex vascular lesion development have been hampered by the fact that plexiform lesions do not develop spontaneously in current animal models of PAH (Stelzner et al., 1992; Rabinovitch, 1997; Rich, 1998; Zabka et al., 2006; Stenmark et al., 2009; Abe et al., 2010; Firth et al., 2010). The Fawn-hooded rat (FHR) spontaneously develops PAH and extensive medial hypertrophy, but spontaneous intimal proliferation and plexogenic arteriopathy have not been observed in the FHR (Kentera et al., 1988; Sato et al., 1992; Le Cras et al., 1999; Nagaoka et al., 2006). Chronic Hypoxia, monocrotaline toxin, pulmonary emboli, pulmonary arterial banding, and hyperdynamic systemic-to-pulmonary artery shunts have been used to induce PAH and medial hypertrophy in various mammalian models. However, the pathogenic mechanisms attributable to these initiating stimuli likely differ from those in spontaneous human IPAH, and none of these models have been found to recapitulate the progressive development of severe, obstructive plexiform lesions similar to those observed in human IPAH (Zamora et al., 1996; Rabinovitch, 1997; Botney, 1999; Tyler et al., 1999; Gust and Schuster, 2001; Taraseviciene-Stewart et al., 2001; Medhora et al., 2002; Stenmark et al., 2009; Firth et al., 2010). Neointimal development can be induced in laboratory animals by imposing multiple experimental challenges to create very severe PAH. For example, occlusive neointimal lesions formed in the pulmonary arterioles of rats when monocrotaline toxin administration was combined with severe hemodynamic stress caused by unilateral pneumonectomy or the creation of an anastomosis between systemic and pulmonary arteries (Tanaka et al., 1996; Botney, 1999; Vaszar et al., 2004; Albada et al., 2005). Neointimal lesions also formed when rats deficient in endothelin type B receptors (ETB) were injected with monocrotaline toxin (Ivy et al., 2005). The active metabolite of monocrotaline toxin directly damages the pulmonary endothelium (Huxtable, 1990; Lame et al., 2000). Endothelial cell proliferation clearly contributes to neointimal obstruction of pulmonary arterioles in combined-challenge animal models (Taraseviciene-Stewart et al., 2001, 2006; Nishimura et al., 2002, 2003). Chronic hypoxia combined with blockade of VEGF receptors triggered the formation of occlusive neointimal lesions in rats (Taraseviciene-Stewart et al., 2001, 2006). Recently the same combined-challenge model (VEGF receptor blockade plus chronic hypoxia) was used to initiate pulmonary hypertension, after which the rats were maintained under normoxic conditions. Severe, progressive pulmonary hypertension ensued, accompanied by complex plexiform-like lesions closely resembling human plexiform lesions (Abe et al., 2010). Plexiform lesions also were detected in a retrospective study of dogs whose tissues had been archived between 1983 and 2003. These dogs had well documented IPAH and the lungs of four individuals contained plexiform lesions that were regionally clustered downstream from branching points of pulmonary arterioles (Zabka et al., 2006). Plexiform lesions also developed in dogs and sheep two to four months after severe PAH was initiated by creating hyperdynamic systemic-to-pulmonary artery shunts in the superior lobe of one lung (Saldana et al., 1968; Schnader et al., 1996). This evidence supports the hypothesis that complex obstructive lesions may fail to develop in most laboratory mammals because the PAP and shear stress are insufficiently elevated, the hyperdynamic forces are not sustained long enough, or the animals succumb too rapidly to severe PAH (Rabinovitch, 1997; Albada et al., 2005; Abe et al., 2010). Recent comprehensive reviews emphasize that many of the current mammalian models of PAH greatly facilitated pioneering advances in understanding and treating human IPAH. However none of these animal models fully replicate the pathogenesis of human IPAH nor, absent multiple challenges, does plexogenic arteriopathy arise spontaneously in the current mammalian models of PAH (Stenmark et al., 2009; Firth et al., 2010).
4.4 Avian Model of Spontaneous IPAH
Anatomical and functional descriptions of the lungs, airways and pulmonary vasculature of domestic fowl have been provided previously by Duncker (1972), Abdalla and King (1975), Maina (1988), and Brown et al. (1997). Chickens bred for rapid growth and meat production (broiler chickens, broilers) provide an excellent model of spontaneous IPAH, encompassing many of the classic symptoms and a genetically tractable system for elucidating the underlying causes. Broiler chicks typically hatch at a weight of 40 g and can grow to 4 kg within 8 weeks. Thus in two months a broiler’s body weight doubles and redoubles almost 7 times. If human infants grew at the same rate, their body weight would increase from 3 kg (6.6 lb) at birth to 310 kg (690 lb) at 2 months of age. The extremely rapid early growth performance of broilers imposes proportional challenges to their developmentally immature pulmonary and cardiovascular systems, thereby triggering a suite of “metabolic diseases” that are attributable primarily to rapid growth rather than to infectious pathogens. Approximately 3% of all broiler chickens spontaneously develop IPAH when reared under conditions that promote maximal growth. Rapid growth incurs corresponding increments in cardiac output that must be propelled through lungs that remain essentially isovolumetric throughout the respiratory cycle, and that are constrained in volume by the dimensions of the dorsal thoracic rib cage (Wideman, 1999, 2000, 2001). The pulmonary vasculature of broilers exhibits low compliance and is fully engorged with blood at a normal (resting) cardiac output, unlike the situation in mammals in which the pulmonary vasculature is compliant and recruitable when cardiac output increases (Peacock et al., 1989; Wideman and Kirby, 1995a,b; Wideman et al., 1996a,b; Wideman, 2000). Total lung volume also is poorly correlated with body mass in broilers, creating an incipient pulmonary hemodynamic insufficiency that continues to be further exacerbated by ongoing genetic selection for rapid muscle accretion and thus increased metabolic demand for O2 (Julian, 1989; Peacock et al., 1989, 1990; Owen et al., 1995a,b; Silversides et al., 1997; Wideman, 1999). These observations serve as the basis for the hypothesis that the pulmonary vascular capacity of broiler chickens is marginally adequate to accommodate the cardiac output required to support fast growth. The pulmonary vascular capacity can be broadly defined to encompass metabolic limitations related to the tone (degree of contraction) maintained by the primary resistance vessels (arterioles), as well as anatomical constraints related to the compliance and effective volume of the blood vessels (Wideman 2000, 2001; Wideman et al., 2007).
Abundant evidence demonstrates that broilers with the most limited pulmonary vascular capacity spontaneously develop IPAH when the right ventricle is forced to develop an excessively elevated PAP to propel the cardiac output through their lungs. Broiler chickens that otherwise appear to be clinically healthy can be demonstrated, by pulmonary arterial catheterization, to have an elevated PAP that precedes characteristic work hypertrophy of the right ventricle (Wideman et al., 2006; 2010a). Wedge pressure measurements confirm the precapillary vasculature as the primary source of excessive resistance to blood flow (Chapman and Wideman, 2001; Lorenzoni et al., 2008; Wideman et al., 2010a). Sustained pulmonary hypertension leads to right ventricular work hypertrophy, as demonstrated by increases in the right-to-total ventricular weight ratios. Following the onset of PAH a distinctive pathophysiological progression includes the development of systemic arterial hypoxemia (cyanosis), polycythemia, systemic arterial hypotension attributable to reduced total peripheral resistance, regurgitation by the monocuspid right atrio-ventricular valve, cardiac decompensation, right-sided congestive heart failure, central venous hypertension, hepatic cirrhosis, transudation of plasma from the liver into the abdominal cavity (ascites), and death. The systemic arterial hypoxemia is attributable to a diffusion limitation initiated by erythrocytes flowing too rapidly past the pulmonary gas exchange surfaces to permit full equilibration with oxygen (Peacock et al., 1990; Wideman et al., 1996a, 2007; Wideman, 2000, 2001). Minimally invasive methods such as electrocardiography and pulse oximetry can be used to identify subclinical/intermediate stages of PAH, including the onset of right ventricular hypertrophy (electrocardiography) and systemic arterial hypoxemia (pulse oximetry and hematocrit) (Wideman, 2000, 2001). Mortality attributable to IPAH can exceed 20% of the broilers subjected to experimental conditions designed to excessively increase the cardiac output or pulmonary vascular resistance, including exposure to sustained sub- or supra-thermoneutral environmental temperatures, chronic hypoxia, respiratory disease, poor air quality, hyperthyroidism, or partial occlusion of the pulmonary vasculature (Cueva et al., 1974; Sillau and Montalvo, 1982; Huchzermeyer and DeRuyck, 1986; Hernandez, 1987; Julian, 1988, 1993; Peacock et al., 1989, 1990; Wideman and Kirby, 1995a,b; Wideman, 1999, 2000, 2001; Wideman et al., 1996a,b, 1997, 2000, 2007).
Procedures used to reveal sub-clinical genetic susceptibility to IPAH within breeding populations of broilers include exposure to hypobaric hypoxia (hypoxic pulmonary vasoconstriction) (Ploog, 1973; Owen et al., 1990, 1995a,b,c; Anthony et al., 2001; Balog, 2003), surgical occlusion of one pulmonary artery (50% reduction in pulmonary vascular capacity) (Wideman and Kirby, 1995a,b; Wideman and French, 1999; 2000), and intravenous microparticle injections (proportional occlusion of pulmonary arterioles and initiation of focal inflammatory responses) (Wideman and Erf, 2002; Wideman et al., 2002; Wang et al., 2003; Hamal et al., 2008, 2010a,b). Exposure to sustained hypobaric hypoxia has been used to select IPAH-susceptible and IPAH-resistant broiler lines for multiple generations (Anthony et al., 2001; Balog, 2003). Broilers from the IPAH-susceptible line succumb to IPAH during exposure to hypobaric hypoxia or cool temperatures, or when microparticles are injected i.v. to partially occlude pulmonary arterioles (vide infra), whereas broilers from the IPAH-resistant line remain markedly unperturbed by these challenges (Wideman et al., 2002; Chapman and Wideman, 2006b). Clinically healthy broilers from the IPAH-susceptible line spontaneously develop higher PAP and PVR when compared with clinically healthy individuals from the IPAH-resistant line (Wideman et al., 2002, 2007; Bowen et al., 2006a,b; Chapman and Wideman, 2006b; Lorenzoni et al., 2008). Lung volume as a percentage of BW does not differ between broilers from these lines (Wideman, unpublished observations). The cumulative evidence therefore demonstrates that selection pressures rigorously focused to challenge the pulmonary vascular capacity readily expose the genetic basis for spontaneous IPAH in broilers. The genes responsible for avian IPAH have a high heritability, estimated at 0.4 to 0.6 (Huchzermeyer et al., 1988; Peacock et al., 1989; Lubritz et al., 1995; Wideman and French, 1999, 2000; Moghadam et al., 2001; Deeb et al., 2002; Pakdel et al., 2002). Attempts to correlate mutations in the chicken BMPR2 with IPAH susceptibility were unsuccessful. Fourteen single nucleotide polymorphisms (SNPs) were identified in broiler BMPR2 mRNA, but no mutations unique to IPAH susceptibility were present, nor were differences in BMPR2 mRNA expression levels detected between susceptible and resistant individuals (Cisar et al., 2003a,b).
4.5 Pulmonary Vascular Pathology in the Avian Model of IPAH
Broilers with IPAH consistently exhibit medial hypertrophy within 25 to 100µ diameter interparabronchial pulmonary arterioles that are homologous to the pre-acinar arteries of mammals (Cueva et al., 1974; Hernandez, 1987; Peacock et al., 1989; Sillau and Montalvo, 1982; Maxwell, 1991; Enkvetchakul et al., 1995; Xiang et al., 2002, 2004; Moreno de Sandino and Hernandez, 2003, 2006; Pan et al., 2005; Tan et al., 2005a,b). Medial hypertrophy has been attributed, in part, to reduced apoptosis of smooth muscle cells in the pulmonary arterioles (Tan et al., 2005b). Intimal proliferation and activation of endothelial protein kinase C (PKCα) also have been observed within the muscularized pulmonary arterioles of broilers developing PAH (Xiang et al., 2002; Tan et al., 2005a,b). However, until recently there were no reports of plexiform lesions in avian lungs. Accordingly, we surveyed the lungs of male and female broilers from an IPAH-susceptible line to estimate the relative age- and gender-specific incidences of plexiform lesion development (Wideman et al., 2010b). We focused on the interparabronchial arterioles (Abdalla and King, 1975) because these key branch points within the avian pulmonary vascular bed appear to be homologous to the arteriole branch sites that promote plexiform lesion development in humans and laboratory mammals (Cool et al., 1999; Zabka et al., 2006; Stenmark et al., 2009; Abe et al., 2010). Plexiform lesions developed in both females and males, and lesion incidences (lung sections with lesions/lung sections examined) averaged approximately 40% in 8 to 52 week old birds. Plexiform lesion densities averaged 0.20 lesions per cm2 which is the lower range of lesion densities (0.1 to 11.7 pe r cm2) reported for human patients confirmed to have plexogenic pulmonary arteriopathy (Wagenvoort et al., 1970; Yamaki and Wagenvoort, 1985). In humans with severe IPAH ongoing lesion development irreversibly obliterates small pulmonary arteries, pruning the pulmonary vasculature and fueling a positive feedback cycle in which the accumulating vascular obstruction is thought to progressively increase the PVR and elevate the right ventricular afterload. The existing hemodynamic challenge is further aggravated when the hypertrophied right ventricle is forced to elevate the PAP to propel the cardiac output through the decreasing numbers of pulmonary blood vessels that remain unobstructed (Naeye and Vennart, 1959; Dammann et al., 1961; Voelkel et al., 1998; Stenmark et al., 2009). The limited extent of plexogenic vascular obstruction in broilers does not seem likely to significantly increase the PVR, and we have not detected age-related increases in plexiform lesion incidences or densities. Accordingly, plexiform lesion development appears to be a consequence rather than the proximate cause of pulmonary hypertension in IPAH-susceptible broilers (Wideman et al., 2010b).
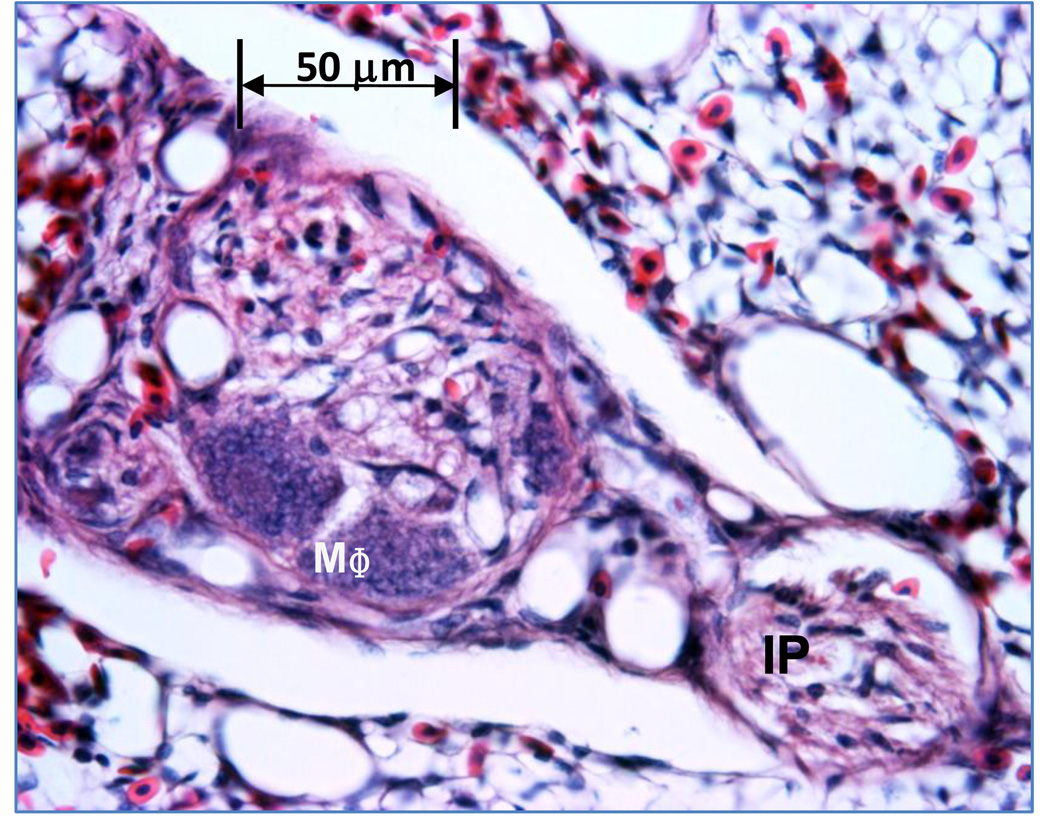
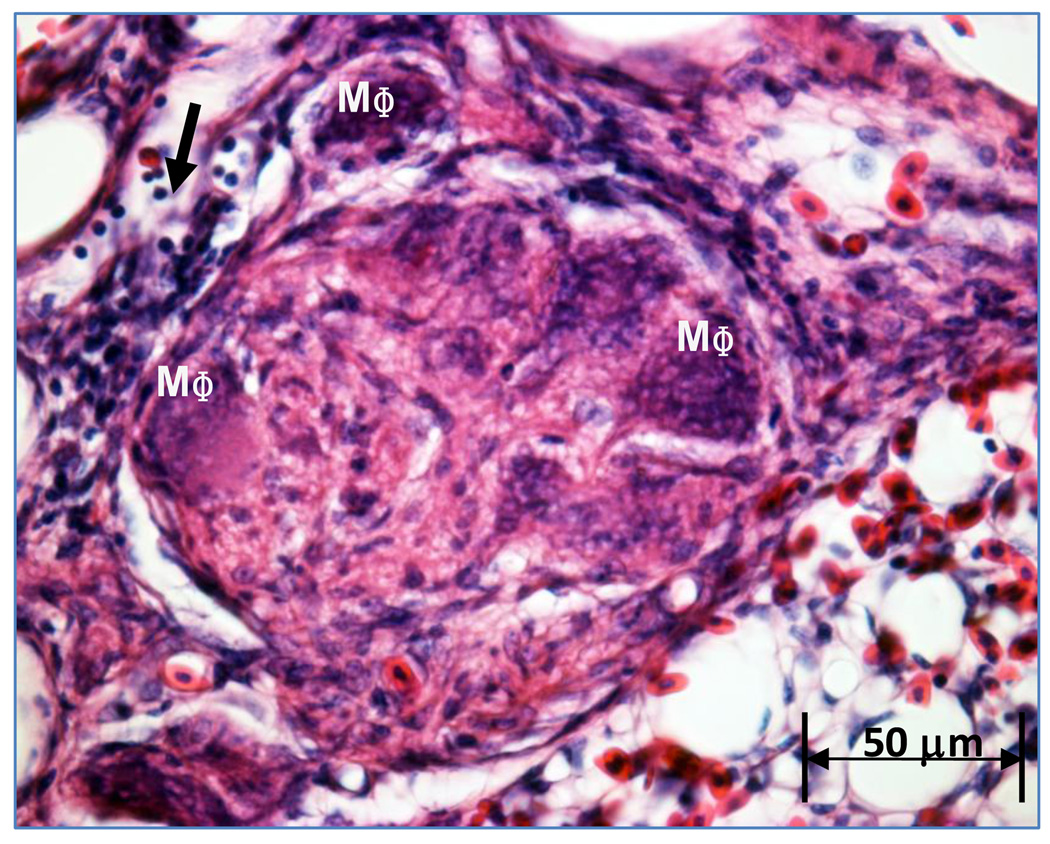
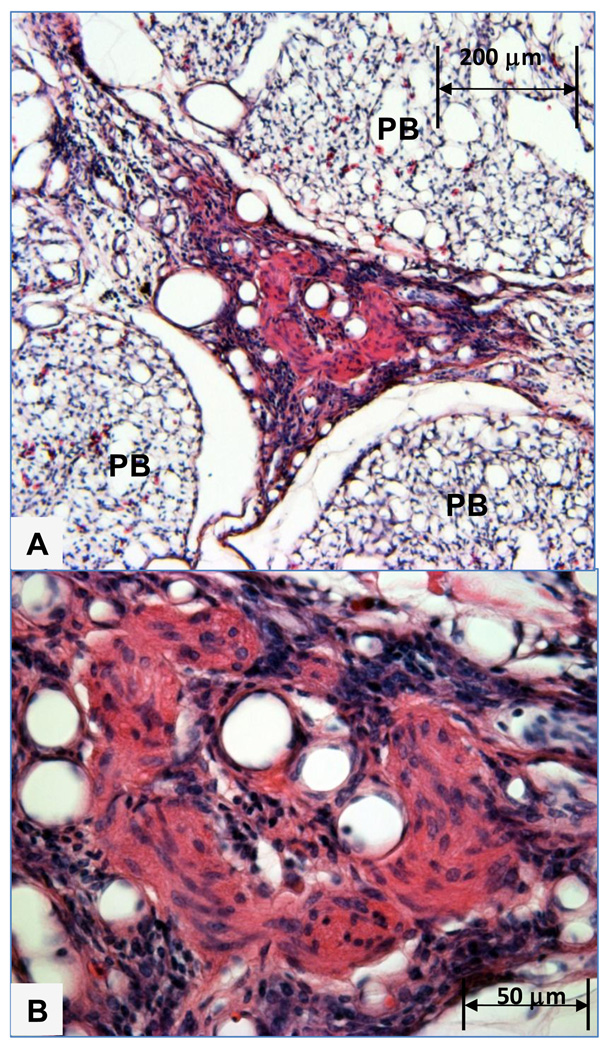
Our observations suggest a maturational process occurs through which compact cellular lesions having a relatively homogeneous matrix and sparse vascular channels transition into larger ‘mature’ plexiform lesions exhibiting numerous vascular channels and multiple cell types including connective tissue, inflammatory cells and proliferating intimal cells. Plexiform lesions are most likely to develop in regions of broiler lungs where perivascular mononuclear cell infiltrates surround interparabronchial arteries at their branch points from parent arteries. Perivascular mononuclear cell infiltrates often occur in the absence of notable inflammation within the adjacent gas exchange parenchyma or airways. Plexiform lesions also are likely to develop in portions of the lung where muscularized pulmonary arteries exhibit cellular intimal proliferation. Well developed medial hypertrophy and intimal proliferation are readily detected but unevenly distributed within broiler lungs. Small, apparently early-stage, plexiform lesions forming within interparabronchial arteries typically consisted of an intimal cellular matrix and embedded macrophages, an apparently un-distended smooth muscle layer, and extramural inflammatory cell infiltrates (Figures 1 and 2). Plexiform lesions have been detected in broiler lungs as early as 7 days post-hatch, clearly forming at branch points of interparabronchial arterioles (Figure 3). Medium sized plexiform lesions typically occur shortly downstream from the point where an interparabronchial artery branches from a larger parent artery, or in supernumerary branches from large conducting arteries. Mononuclear cell infiltrates typically are evident around the perimeter of the lesions and nearby arterial branches. Macrophages having a foam-type appearance are arrayed adjacent to the remnants of the dilated arterial wall, surrounding a compact, fairly homogeneous core of intimal cells (Figure 4). In ‘maturing’ plexiform lesions the glomeruloid-like main body (120 to 200 µm in diameter) undergoes aneurysmal expansion well beyond the circumference of a typical interparabronchial artery, foam-type macrophages reside at the periphery of the main body, and remnants of smooth muscle in the arterial wall are scarce or absent (Figures 5 and 6). Perivascular mononuclear cell infiltrates persist in surrounding the parent arteriole, and inflammatory cell infiltrates also may surround the dilated margins of mature plexiform lesions in broiler lungs (Figures 6 and 7). Similar inflammatory processes have been associated with IPAH and plexogenic arteriopathy in humans and laboratory mammals (Caslin et al., 1990; Tuder et al., 1994; Chazova et al., 1995; Cool et al., 1997; Dorfmüller et al., 2002; Nicolls et al., 2005; Zabka et al., 2006; Stenmark et al., 2009). It remains to be determined if vascular injury induced by shear stress attracts lymphocytes to areas of lesion formation, or if lymphocytes responding to injury contribute to changes in intimal and medial cells that promote the development of plexiform lesions (Dorfmüller et al., 2002, 2003; Nicolls et al., 2005).
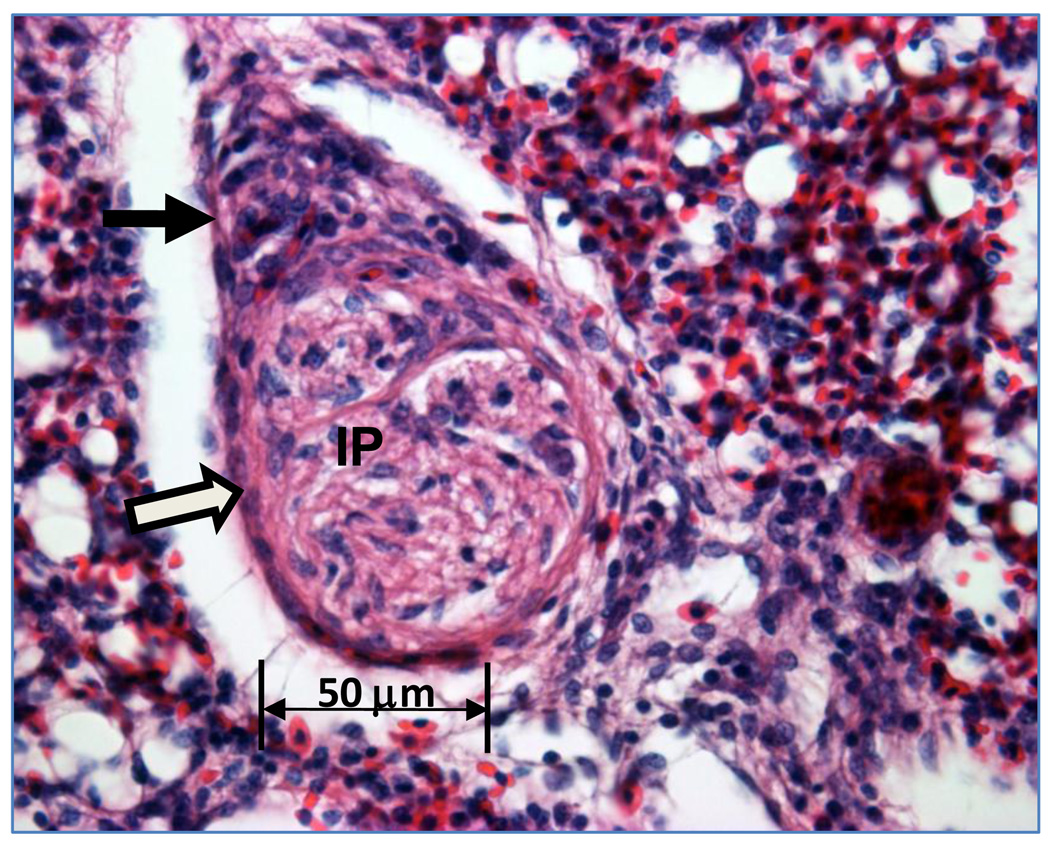
Figure 1.
Section through an interparabronchial arteriole reveals intimal proliferation (IP) within the arteriole lumen. Closed arrow indicates perivascular mononuclear cells outside the smooth muscle wall of the arteriole (open arrow). Hematoxylin and eosin staining in all figures.
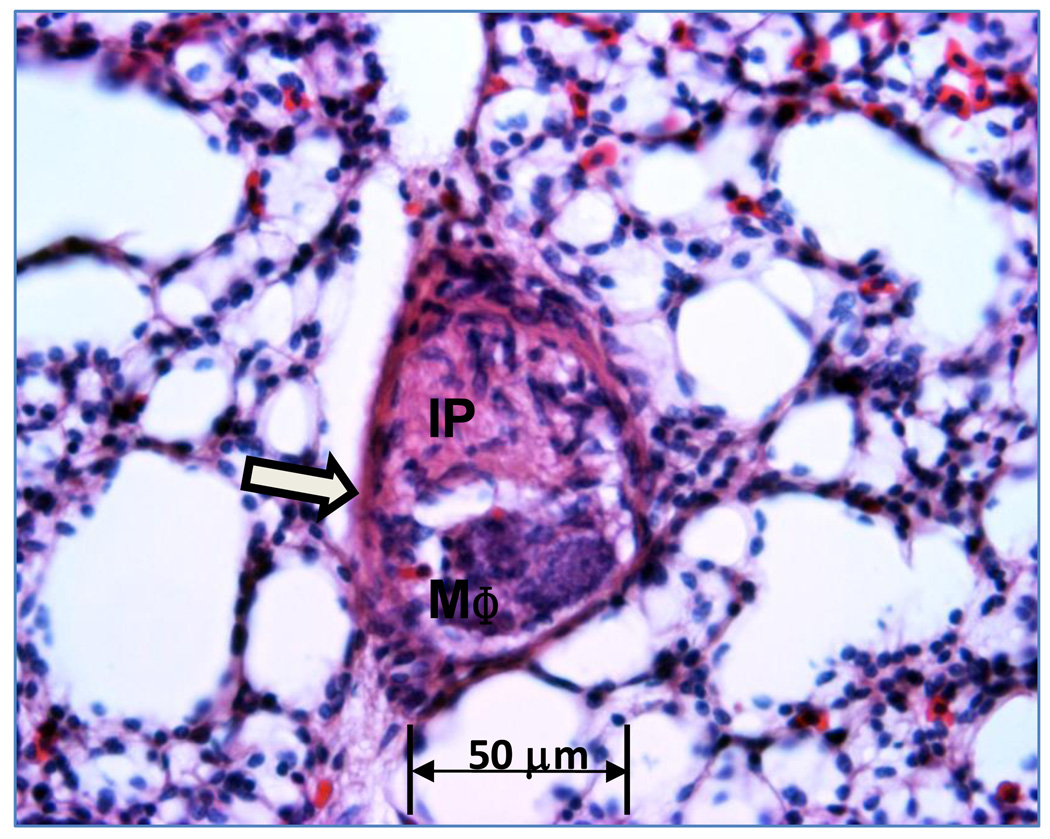
Figure 2.
An arteriole with the lumen occluded by proliferating intimal cells (IP) and macrophages (MΦ). Sparse perivascular mononuclear cells surround the arteriole wall (open arrow).
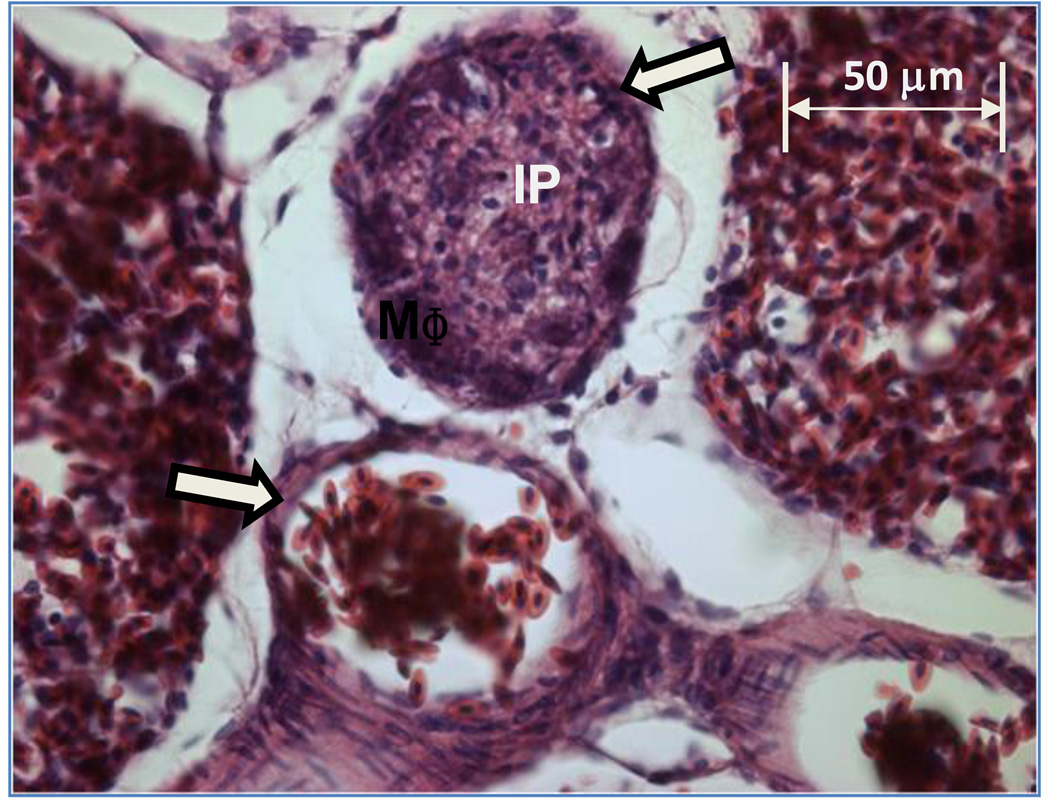
Figure 3.
A small plexiform lesion in a branch of an interparabronchial arteriole exhibits macrophages (MΦ) arrayed inside the remnants of the smooth muscle wall (open arrow), with proliferating intimal cells (IP) occluding the lumen. Adjacent branches of the same interparabronchial arteriole contain nucleated avian erythrocytes within the smooth muscle wall (open arrow). This lung from a 7 day old broiler chick was fixed by immersion in phosphate buffered formalin.
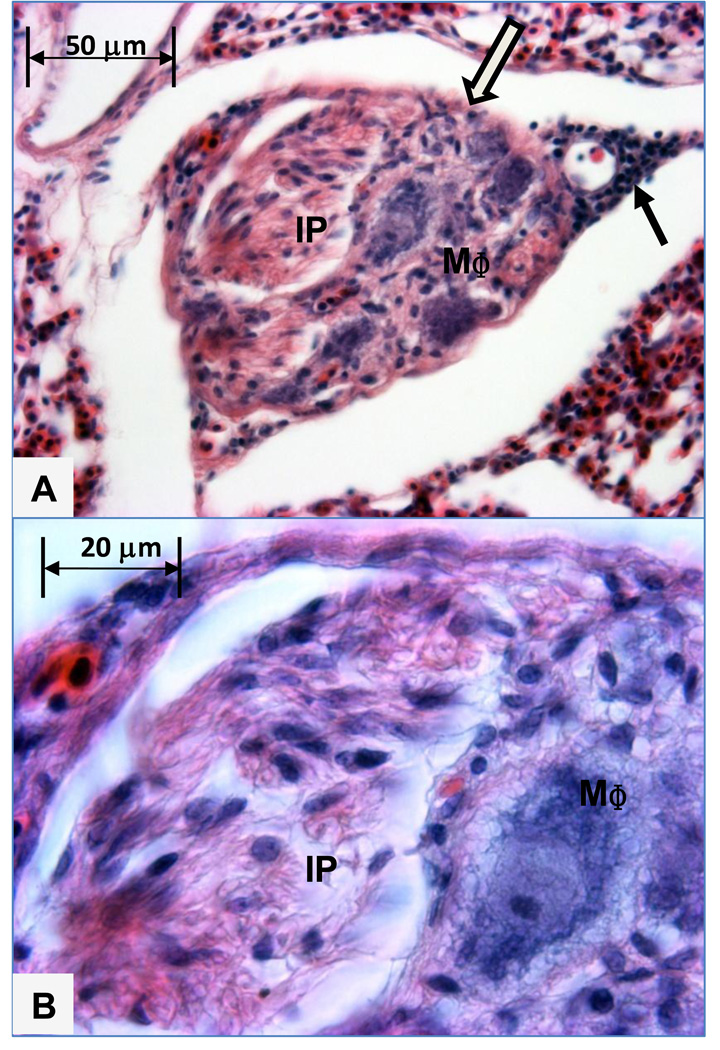
Figure 4.
A. This intermediate sized plexiform lesion developed in an interparabronchial arteriole branch coursing within the septum between adjacent parabronchi. The lesion contains foam-type macrophages (MΦ) adjacent to the remnant of the arteriole wall on the bottom right and a channel filled intimal proliferating cells (IP) adjacent to the arteriole smooth muscle (open arrow) on the upper left. Filled arrow indicates perivascular mononuclear cells outside the wall of the arteriole. Figure 4B. Higher magnification shows the intimal proliferating cells (IP) and a foam-type macrophage (MΦ).
Figure 5.
Maturing plexiform lesions contain foam-like macrophages (MΦ) at the periphery near the remnants of the arteriole wall, with the core of the lesion becoming glomeruloid in form due to penetration by multiple vascular channels. A swath of intimal proliferating cells (IP) courses within the lumen of an adjacent segment of the arteriole.
Figure 6.
This plexiform lesion exhibits a central matrix of intimal proliferating cells penetrated by vascular channels, with multiple foam-type macrophages (MΦ) arrayed adjacent to the indistinct remnants of the arteriole’s wall. Filled arrow indicates mononuclear cells.
Figure 7.
A. Mature plexiform lesion in a 24 week old male broiler evidently developed in an interparabronchial arteriole coursing between three parabronchi (PB). The central matrix of the lesion contains epithelioid cells and myofibroblasts, and is penetrated by vascular channels that were preserved in an open configuration by in situ trans-cardiac perfusion fixation. Figure 7B. Higher magnification shows vascular channels penetrating the central core of the lesion, which is surrounded by perivascular mononuclear cell infiltrates.
4.6 Role of Serotonin (5-HT) in IPAH and Vascular Remodeling
Serotonin (5-HT) has been implicated in the pathogenesis of IPAH (Hervé et al., 1995; MacLean et al., 2000; Eddahibi et al., 2001a, 2002; Marcos et al., 2003, 2004; Naeije and Eddahibi, 2004; Eddahibi and Adnot, 2005; Lawrie et al., 2005), PAH triggered by serotonergic appetite suppressant drugs (Seiler et al., 1974; Douglas et al., 1981; Brenot et al., 1993a,b; Abenhaim et al., 1996; Louis, 1999; Eddahibi et al., 2001b; Eddahibi and Adnot, 2002; Naeije and Eddahibi, 2004), PAH initiated by hypoxia and monocrotaline toxin (Demiryurek et al., 1991; Uzun et al., 1998; Eddahibi et al., 1997, 1999, 2000; Marcos et al., 2003; Guignabert et al., 2005), and PAH associated with Gram-negative sepsis, ARDS, COPD, and other inducers of secondary PAH in humans (Sibbald et al., 1980; Heffner and Repine, 1997; Egermayer et al., 1999; MacLean et al., 2000; Eddahibi et al., 2003). Serotonin initiates rapid and sustained increases in PVR, reflecting acute vasoconstriction followed by structural remodeling associated with smooth muscle cell proliferation. Acute pulmonary vasoconstriction is mediated by 5-HT2A, 5-HT2B, and 5-HT1B/1D receptors expressed on vascular smooth muscle cells (McGoon and Vanhoutte, 1984; Choi and Maroteaux, 1996; MacLean et al., 1996, 2000; Morecroft et al., 1999; Keegan et al., 2001; Lawrie et al., 2005; Cogolludo et al., 2006; Rodat-Despoix et al., 2008). Serotonin receptor expression did not appear to be altered in one evaluation of patients with IPAH (Marcos et al., 2004), but increased 5-HT1B and 5-HT2B receptor expressions have been implicated in other studies of human IPAH (Morecroft et al., 1999; Launay et al., 2002). Structural remodeling is mediated by the high affinity serotonin transporter (5-HTT, SERT) that primarily is expressed by pulmonary vascular smooth muscle but also is expressed by the endothelium. Serotonin translocated into the smooth muscle cells by 5-HTT is mitogenic, causing hypertrophy and proliferation of the medial muscle layer in pulmonary arterioles (Lee et al., 1991, 1994; Ramamoorthy et al., 1993; Pitt et al., 1994; Zamora et al., 1996; Fanburg and Lee, 1997; Eddahibi et al., 1999; Marcos et al., 2003, 2004). 5-HTT expression and smooth muscle cell proliferation mediated by serotonin are markedly amplified in lung tissues, pulmonary arteries, and platelets from human IPAH patients (Eddahibi et al., 2001a, 2002). Multi-fold increases in 5-HTT gene transcription and 5-HTT expression in IPAH patients have been attributed to the L allele of the 5-HTT gene promoter. The homozygous (LL) genotype appears to confer susceptibility to IPAH when combined with other predisposing factors, including the BMPR2 gene mutation (Heils et al., 1996; Lesch et al., 1996; Eddahibi et al., 2001a, 2002; Du et al., 2003; Sullivan et al., 2003; Eddahibi and Adnot, 2005). Thus, mice with targeted disruption (knockout) of the BMPR2 gene and their wild-type littermates did not exhibit differences in pulmonary hemodynamics or vascular morphometry under normoxic or hypoxic conditions until serotonin was infused chronically, after which the BMPR2 deficient mice developed markedly amplified PAH and vascular remodeling (Long et al., 2006). The LL genotype of the 5-HTT gene promoter also predisposes human COPD patients to more severe PAH and vascular remodeling (Eddahibi et al., 2003).
Competitive inhibitors of 5-HTT but not 5-HT1B/1D receptor antagonists eliminate the ability of serotonin and its agonists to stimulate human pulmonary artery smooth muscle cell proliferation in vitro (Eddahibi et al., 2001a; Marcos et al., 2003). Monocrotaline toxin increases 5-HTT expression, vascular remodeling and PAH, whereas subsequent inhibition of 5-HTT completely reverses the PAH and vascular remodeling induced by monocrotaline toxin (Guignabert et al., 2005). Direct evidence of the role played by 5-HTT in pulmonary vascular remodeling has been demonstrated in 5-HTT gene knockout mice, and in mice treated with 5-HTT competitive inhibitors. Indices of PAH (right ventricular hypertrophy, elevated right ventricular systolic pressure) and arteriole muscularization were attenuated in mice lacking functional 5-HTT when compared with the PAH responses of wild-type or vehicle-injected control mice during chronic (2 week) exposure to normobaric hypoxia or following monocrotaline toxin injection (Eddahibi et al., 2000; Marcos et al., 2003). Conversely, transgenic mice overexpressing 5-HTT predominantly in vascular smooth muscle cells developed marked PAH, right ventricular hypertrophy and muscularization of distal pulmonary arterioles without changes in systemic arterial pressure (Guignabert et al., 2006). Chronic hypoxia triggers increased 5-HTT expression leading to enhanced smooth muscle cell proliferation and PAH in response to serotonin supplementation (Eddahibi et al., 1999, 2001b). Paradoxically, competitive inhibition of 5-HTT potentiated the PAH response of mice exposed to acute (5 minute) normobaric hypoxia when compared with vehicle-treated controls. The latter observations are consistent with the hypothesis that inhibiting serotonin uptake by platelets increased the plasma pool available to bind to serotonin receptors on smooth muscle cells, resulting in amplified pulmonary vasoconstriction during the acute PAH response to hypoxia. Co-treatment with competitive 5-HTT inhibitors plus 5-HT1B receptor antagonists blunted the PAH response to acute hypoxia (Marcos et al., 2003), and 5-HT1B receptor knockout mice were less susceptible to the onset of hypoxic PAH and vascular remodeling than were wild-type controls (Keegan et al., 2001; Launay et al., 2002). The 5-HT1B receptor and 5-HTT interact to regulate Rho kinase-mediated pulmonary vascular remodeling (Liu et al., 2004; Hyvelin et al., 2005), and interactions between 5-HT1B receptors and 5-HTT regulate the S100A4/Mts1 gene that is induced in clinical IPAH (Lawrie et al., 2005). These studies reemphasize the importance of 5-HTT expression and co-dependent interactions with serotonin receptors in the chronic pathogenesis of PAH (Marcos et al., 2003; Guignabert et al., 2005; Lawrie et al., 2005).
An important linkage between plexiform lesions and serotonin can be deduced from the pathogenesis of IPAH triggered by anorexigens (appetite suppressants) such as aminorex, dexfenfluramine and the combination of fenfluramine and phentermine (fen/phen). In a small percentage of anorexigen users severe PAH evolved with a latency of up to 23 years. The histopathological, morphological and clinical features were considered indistinguishable from those in familial IPAH, including medial hypertrophy and the formation of plexiform lesions in pulmonary arterioles (Tuder et al., 1998a,b). Endothelial cells from plexiform lesions tend to be monoclonal in origin when obtained from patients with either anorexigen-triggered PAH or spontaneous IPAH, but polyclonal in origin when obtained from patients with secondary PAH (Lee et al., 1998; Tuder et al., 1998a,b). Anorexigens are serotonergic and are thought to trigger IPAH by: stimulating serotonin release from cellular stores such as nerve terminals and platelets; inhibiting 5-HTT and thus serotonin uptake by platelets and neurons; increasing plasma serotonin levels; and, directly stimulating serotonin receptors (Seiler et al., 1974; Celada et al., 1994; Abenhaim et al., 1996; Weir et al., 1996; DeJonghe and Swinkels, 1997; Rothman et al., 1999; Louis, 1999; Russell and Laverty, 2000; Eddahibi et al., 2001a, 2001b; Eddahibi and Adnot, 2002; Launay et al., 2002). Individuals may be particularly susceptible to anorexigen-related IPAH when the presence of the LL genotype of the 5-HTT promoter imparts high basal level of serotonin uptake by pulmonary vascular smooth muscle cells (Eddahibi et al., 2001a).
Mechanisms by which serotonin and anorexigens contribute to endothelial proliferation and plexiform lesion formation remain to be clarified. Serotonin is actively transported into endothelial cells and endothelial serotonin uptake is stimulated by hypoxia (Lee and Fanberg, 1986a,b, 1987). Human pulmonary endothelial cells exhibit 5-HTT and 5-HT2B-receptor immunoreactivity, and 5-HTT mRNA expression was observed in cultured endothelial cells from patients with non-familial IPAH but not in endothelial cells from control subjects (Eddahibi et al., 2001a, 2006; Marcos et al., 2004). Active serotonin uptake by the pulmonary endothelium may primarily serve to clear serotonin from the circulation (Fanberg and Lee, 1997). However serotonin also is mitogenic for isolated endothelial cells, leading to the proposal that platelets aggregating at sites of endothelial damage may release serotonin in quantities sufficient to induce endothelial proliferation (Pakala et al., 1994). The development of thrombogenic surfaces in response to elevated shear stress likely promotes in situ thrombosis and platelet aggregation within the pulmonary arterioles of patients with IPAH. Platelets and the endothelium to which they adhere can release of a variety of mitogens (5-HT, ET-1, PAF, PDGF, VEGF, FGFa, TGF- β) that potentially can stimulate endothelial and smooth muscle cell proliferation (Farber and Loscalzo, 1999; Humbert et al., 2004; Hoeper et al., 2006). Serotonin is the most potent mitogen of all the endothelial- and platelet-derived growth factors evaluated in isolated pulmonary arterial smooth muscle cell cultures (Eddahibi et al., 2001a; 2006). Evaluations of pulmonary vascular remodeling have focused on paracrine (cross-talk) pathways involving serotonin, smooth muscle cells and the endothelium. Vascular smooth muscle cells produce the protein angiopoietin 1 (Ang-1) which promotes angiogenesis by binding to TIE2 receptors that are localized exclusively on endothelial cells. Ang-1 activation of TIE2 receptors stimulates pulmonary endothelial cells to synthesize and secrete serotonin, which in turn triggers vascular remodeling. Extremely strong positive correlations (r = 0.97) have been demonstrated between pulmonary Ang-1 expression, TIE2 activation, and PVR in patients with non-familial IPAH (Du et al., 2003; Sullivan et al., 2003). Quiescent pulmonary endothelial cells from patients with non-familial IPAH also synthesize and secrete more serotonin and exhibit markedly upregulated expression of tryptophan hydroxylase (TPH), the rate-limiting enzyme in serotonin biosynthesis, when compared with control endothelial cells. TPH immunoreactivity was confined to the intima and was not observed in smooth muscle cells in tissues from IPAH patients with marked medial hypertrophy (Eddahibi et al., 2006). These studies provide a plausible linkage between dysregulated Ang-1 or TPH gene expression and the involvement of serotonin in the increased PVR and associated vascular remodeling that are characteristic of IPAH.
4.7 Serotonin is a Key Vasoconstrictor in Avian IPAH
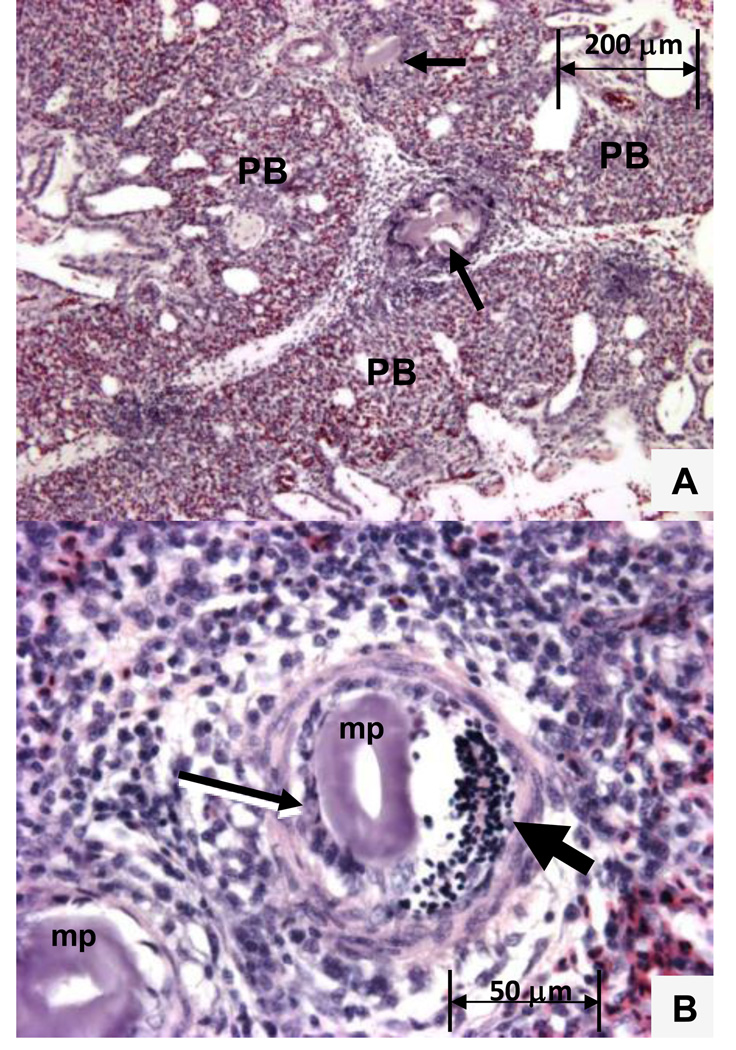
A primary role for serotonin in avian IPAH was revealed through the use of the i.v. microparticle injection technique. Microparticles having a size suitable for occluding arterioles are injected into a systemic vein to be carried to the lungs by the returning venous blood. Pulmonary arterioles become occluded in proportion to the number and size of the microparticles injected, thereby increasing the PVR and triggering acute physiological responses that mirror those previously observed following acute unilateral pulmonary artery occlusion and that are characteristic of broilers that spontaneously develop IPAH (Wideman and Erf, 2002; Wideman et al., 2005, 2006). As shown in Figure 8, entrapped microparticles also trigger a marked focal pulmonary inflammatory response that includes the perivascular infiltration of mononuclear cells in combination with luminal accumulations of thrombocytes and macrophages (Wideman and Erf, 2002, Wideman et al., 2002, 2003, 2005, 2006, 2007; Wang et al., 2003; Hamal et al., 2008; 2010a,b). Microparticle entrapment elicits higher chemokine and cytokine expression in the lungs of IPAH-resistant than in IPAH-susceptible broilers (Hamal et al., 2010a). Thrombocytes rapidly aggregate around microparticles lodged in the lumen of inter-parabronchial arterioles (Wideman et al., 2002, 2004, 2007; Wang et al., 2003). Thrombocytes are the most numerous leukocytes in avian blood and are functional homologs of mammalian platelets. Thrombocytes and platelets accumulate serotonin within intracellular storage granules that are released upon activation (Kuruma et al., 1970; Simoneit et al., 1970; Sorimachi et al., 1970, 1974; Lacoste-Eleaume et al., 1994). Microparticle injections more than double plasma serotonin levels in broilers (Chapman et al., 2008). Serotonin increases the PVR and PAP in broilers, and is singularly the most potent pulmonary vasoconstrictor we have evaluated. Serotonin is capable of triggering an essentially instantaneous and fully obstructive vasoconstriction leading to an immediate >90% reduction in cardiac output and terminal suffocation within 30 seconds in clinically healthy broilers unless i.v. infusion rates are carefully titrated to 100-fold lower than levels typically used to elicit PAH in mammals (Chapman and Wideman, 2002). The pulmonary hemodynamic responses to serotonin were evaluated in broilers pretreated with the selective 5-HT2A receptor antagonist ketanserin or with the nonselective 5-HT1/2 receptor antagonist methiothepin (Engel et al., 1986; Barnes and Sharp, 1999). Pre-treating broilers with ketanserin failed to alter the PAH response to subsequent serotonin infusion, whereas pretreatment with methiothepin reduced PAP below baseline values and virtually eliminated increases in PVR and PAP elicited by i.v. infusions of serotonin. Methiothepin clearly blocks serotonin-mediated increases in PVR and PAP in broilers, although the specific receptor subtype involved remains to be determined (Chapman and Wideman, 2006a). In a subsequent study methiothepin was used to evaluate the role of serotonin in the onset of PAH triggered by i.v. microparticle injections (Chapman and Wideman, 2006b). Pretreatment with methiothepin reduced PAP below baseline values, demonstrating serotonin likely exerts tonic control of PVR. Microparticle injections increased PAP by 90% in control broilers, but the same dose of microparticles failed to significantly elevate PAP in broilers that had been pretreated with methiothepin.
Figure 8.
A. Intrapulmonary inflammatory response 20 minutes after cellulose microparticles were injected i.v. Arrows show microparticles entrapped in interparabronchial arterioles coursing between three parabronchi (PB). Figure 8B. Entrapped microparticles (MP) attract numerous macrophages (thin arrow) and a swarm of nucleated thrombocytes (thick arrow).
Subsequent to injecting a suitable dose of microparticles, broilers with the most limited pulmonary vascular capacity rapidly succumb to respiratory insufficiency whereas those having a sufficiently robust pulmonary vascular capacity thrive as clinically healthy/resistant survivors. Broiler lines selected for IPAH-resistance are substantially more resistant to microparticle injections when compared with their respective unselected (Base) populations or IPAH-susceptible lines (Wideman et al., 2002, 2007). Injecting a high dose of microparticles into broilers from the IPAH-susceptible line elicited 78% mortality in controls as compared with only 20% in those pretreated with methiothepin. Injecting the same microparticle dose into the IPAH-resistant line elicited 12% mortality in controls as compared with zero mortality in those pretreated with methiothepin (Chapman and Wideman, 2006b).
We also evaluated the impact of i.v. microparticle injections on the incidence of plexiform lesions in the lungs of susceptible broilers. Three months after an LD50 injection, some microparticles remained entrapped in the lungs of the survivors, however no plexiform lesions were observed in the vicinity of persisting microparticles. Instead these survivors exhibited a significant overall reduction in their plexiform lesion incidence when compared with the incidence for age-matched controls. Evidently the broilers that succumbed to the microparticle injection were more susceptible than the survivors to plexogenic arteriopathy (Wideman et al., 2010b). Microparticle injections likely serve to eliminate individuals having excessive serotonin biosynthesis, impaired thrombocyte uptake or enhanced release or leakage of serotonin, enhanced receptor-mediated vasoconstrictive responsiveness to serotonin, or altered internalization of serotonin by 5-HTT. Microparticle entrapment evokes patterns of gene expression consistent with our hypothesis that susceptibility to IPAH in broilers is a consequence an anatomically inadequate pulmonary vascular capacity combined with functional predominance of vasoconstriction attributable to serotonin over vasodilation and immune modulation attributable to NO (Wideman et al., 2002, 2004, 2007; Hamal et al., 2008, 2010a,b)
4.8 Summary and Conclusions
Plexiform lesions elevate the pulmonary vascular resistance PVR) by progressively occluding pulmonary arterioles in up to 80% of the human patients with idiopathic pulmonary arterial hypertension (IPAH). Patients with plexogenic arteriopathy tend to be unresponsive to vasodilator therapy and have a poor prognosis for survival. Research is needed to understand and treat plexogenic arteriopathy, but advances have been hindered by the absence of spontaneously developing plexiform lesions in most mammalian models of PAH. Chickens bred for meat production (broiler chickens, broilers) provide an excellent model of spontaneous, genetically based IPAH triggered by increases in PVR attributable in part to serotonin. Serotonin is a potent vasoconstrictor and mitogen known to stimulate the proliferation of vascular endothelial and smooth muscle cells in mammals. Serotonin has been directly linked to the pathogenesis of IPAH in humans, including IPAH linked to serotonergic anorexigens that trigger the formation of plexiform lesions indistinguishable from those observed in other forms of primary IPAH. The mechanisms by which serotonin and anorexigens contribute to endothelial proliferation and plexiform lesion formation remain to be clarified. The onset of IPAH in broiler chickens clearly involves perturbations of the pre-capillary vasculature (e.g., elevated arteriole resistance and vascular remodeling). In this regard, although extreme differences exist between mammals and birds with regard to the anatomy of air passageways, gas exchange compartments, and the processes involved in ventilation, nevertheless the pre-capillary pulmonary vasculature of mammals and birds exhibits striking structural and functional similarities. Moreover, key similarities clearly exist when the avian and mammalian vascular beds and hemodynamic profiles are compared during the pathogenesis of IPAH. The broiler chicken model of IPAH constitutes the only animal model that develops plexogenic arteriopathy spontaneously. Caveats regarding the use of this model include the relatively low plexiform lesion incidences and densities in most birds, as well as the apparent absence of progressively increasing lesion densities (and associated increases in PVR) as the birds age. Progressive, irreversible pruning of the pulmonary vasculature is a critical problem associated with plexogenic arteriopathy in humans suffering from severe IPAH. Our studies have focused on the spontaneous development of plexogenic arteriopathy in rapidly growing broilers reared under optimal (non-challenging) environmental conditions. Under these fairly benign circumstances perhaps relatively few plexiform lesions develop because the PAP and shear stresses are insufficiently elevated and/or are not sustained long enough. It also is possible that the most susceptible broilers succumb too rapidly to terminal right-sided congestive heart failure. The development of plexiform lesions shortly post-hatch (Figure 3) coincides with a period of rapid lung development in concert with equally challenging increases in body mass and cardiac output. Younger birds are easier to maintain and may present an opportunity to obtain escalated lesion incidences and densities in response to appropriate environmental challenges designed to trigger sustained increases in PAP. Higher lesion incidences are desirable if an animal model is to be useful for assessing therapeutic treatments. Alternatively, it is evident that the immune system plays an important role in the pathogenesis of plexiform lesion development. Plexiform lesions may prove to be triggered primarily by vascular damage and the initiating or accompanying immunological responses, rather than by elevated pressure or shear stress per se. Identifying the processes involved in the pathogenesis of IPAH and plexogenic arteriopathy in the broiler model likely will provide key insights that we hope will prove to be relevant to the pathogenesis of plexogenic arteriopathy and IPAH in humans
Acknowledgements
Supported by NIH/National Heart Lung Blood Institute Grant 1R15HL092517 01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdalla MA, King AS. The functional anatomy of the pulmonary circulation of the domestic fowl. Respiration Physiology. 1975;23:267–290. doi: 10.1016/0034-5687(75)90078-x. [DOI] [PubMed] [Google Scholar]
- Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. New England Journal of Medicine. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- Aird WC. Endothelial cell heterogeneity. Critical Care Medicine. 2003;31:S221–S230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- Albada ME, Schoemaker RG, Kemna MS, Cromme-Dijkhuis AH, Van Veghel R, Berger RMF. The role of increased pulmonary blood flow in pulmonary hypertension. European Respiratory Journal. 2005;26:487–493. doi: 10.1183/09031936.05.00015405. [DOI] [PubMed] [Google Scholar]
- Anthony NB, Balog JM, Hughes JD, Stamps L, Cooper MA, Kidd BD, Liu X, Huff GR, Huff WE, Rath NC. Genetic selection of broiler lines that differ in their ascites susceptibility 1. Selection under hypobaric conditions. Proceedings 13th European Symposium on Poultry Nutrition; Blankenberge; Belgium. 2001. pp. 327–328. [Google Scholar]
- Archer S, Rich S. Primary pulmonary hypertension: A vascular biology and translational “work in progress”. Circulation. 2000;102:2781–2791. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- Archer SL, Wu X-C, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explain regional heterogeneity in hypoxic pulmonary vasoconstriction: Ionic diversity in smooth muscle cells. Circulation Research. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- Balog JM. Ascites syndrome (pulmonary hypertension syndrome) in broiler chickens: are we seeing the light at the end of the tunnel? Avian and Poultry Biology Reviews. 2003;14:99–126. [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barst RJ. Pharmacologically induced pulmonary vasodilation in children and young adults with primary pulmonary hypertension. Chest. 1986;89:497–503. doi: 10.1378/chest.89.4.497. [DOI] [PubMed] [Google Scholar]
- Berger RM, Geiger R, Hess J, Bogers AJJC, Mooi WI. Altered arterial expression patterns of inducible and endothelial nitric oxide synthase in pulmonary plexogenic arteriopathy caused by congenital heart disease. American Journal of Respiratory and Critical Care Medicine. 2001;163:1493–1499. doi: 10.1164/ajrccm.163.6.9908137. [DOI] [PubMed] [Google Scholar]
- Botney MD. Role of hemodynamics in pulmonary vascular remodeling. American Journal of Respiratory and Critical Care Medicine. 1999;159:361–364. doi: 10.1164/ajrccm.159.2.9805075. [DOI] [PubMed] [Google Scholar]
- Bowen OT, Erf GF, Anthony NB, Wideman RF. Pulmonary hypertension triggered by lipopolysaccharide (LPS) in ascites-susceptible and -resistant broilers is not amplified by aminoguanidine, a specific inhibitor of inducible nitric oxide synthase (iNOS) Poultry Science. 2006a;85:528–536. doi: 10.1093/ps/85.3.528. [DOI] [PubMed] [Google Scholar]
- Bowen OT, Wideman RF, Anthony NB, Erf GF. Variation in the pulmonary hypertensive responsiveness of broilers to lipopolysaccharide (LPS) and innate variation in nitric oxide (NO) production by mononuclear cells? Poultry Science. 2006b;85:1349–1363. doi: 10.1093/ps/85.8.1349. [DOI] [PubMed] [Google Scholar]
- Brenot F, Hervé P, Petitpretz P. Primary pulmonary hypertension and the appetite suppressant fenfluramine. British Heart Journal. 1993a;89:117–120. doi: 10.1136/hrt.70.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenot F, Hervé P, Petitpretz P, Parent F, Duroux P, Simmoneu G. Primary pulmonary hypertension and fenfluramine use. British Heart Journal. 1993b;70:537–541. doi: 10.1136/hrt.70.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Brain JD, Wang N. The avian respiratory system: a unique model for studies of respiratory toxicosis and for monitoring air quality. Environmental Health Perspectives. 1997;105:188–200. doi: 10.1289/ehp.97105188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- Caslin AW, Heath D, Madden B, Yacoub M, Gosney JR, Smith P. The histopathology of 36 cases of plexogenic pulmonary arteriopathy. Histopathology. 1990;16:9–19. doi: 10.1111/j.1365-2559.1990.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Celada P, Martin F, Artigas F. Effects of chronic treatment with dexfenfluramine on serotonin in rat blood, brain, and lung tissue. Life Sciences. 1994;55:1237–1243. doi: 10.1016/0024-3205(94)00663-6. [DOI] [PubMed] [Google Scholar]
- Chapman ME, Wideman RF. Pulmonary wedge pressures confirm pulmonary hypertension in broilers is initiated by an excessive pulmonary arterial (precapillary) resistance. Poultry Science. 2001;80(4):468–473. doi: 10.1093/ps/80.4.468. [DOI] [PubMed] [Google Scholar]
- Chapman ME, Wideman RF. Hemodynamic responses of broiler pulmonary vasculature to intravenously infused serotonin. Poultry Science. 2002;81:231–238. doi: 10.1093/ps/81.2.231. [DOI] [PubMed] [Google Scholar]
- Chapman ME, Wideman RF. Evaluation of the serotonin receptor blockers ketanserin and methiothepin on the pulmonary hypertensive responses of broilers to intravenously infused serotonin. Poultry Science. 2006a;85:777–786. doi: 10.1093/ps/85.4.777. [DOI] [PubMed] [Google Scholar]
- Chapman ME, Wideman RF. Evaluation of the serotonin receptor blocker Methiothepin in broilers injected intravenously with lipopolysaccharide and microparticles. Poultry Science. 2006b;85:2222–2230. doi: 10.1093/ps/85.12.2222. [DOI] [PubMed] [Google Scholar]
- Chapman ME, Taylor RL, Jr, Wideman RF. Analysis of plasma serotonin levels and hemodynamic responses following chronic serotonin infusion in broilers challenged with bacterial lipopolysaccharide and microparticles. Poultry Science. 2008;87:116–124. doi: 10.3382/ps.2007-00160. [DOI] [PubMed] [Google Scholar]
- Chazova I, Loyd JE, Zhdanov VS, Newman JH, Belenkov Y, Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. The American Journal of Pathology. 1995;146:389–397. [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Maroteaux L. Immunohistochemical localization of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. Federation of European Biochemical Society Letters. 1996;391:45–51. doi: 10.1016/0014-5793(96)00695-3. [DOI] [PubMed] [Google Scholar]
- Cisar CR, Balog JM, Anthony NB, Donoghue AM. Sequence analysis of bone morphogenetic protein receptor type II mRNA from ascitic and nonascitic commercial broilers. Poultry Science. 2003a;82:1494–1499. doi: 10.1093/ps/82.10.1494. [DOI] [PubMed] [Google Scholar]
- Cisar CR, Balog JM, Okiomoto R, Anthony NB, Donoghue AM. The chicken bone morphogenetic protein receptor type II (BMPR2) gene maps to chromosome 7. Animal Genetics. 2003b;34:475–476. doi: 10.1046/j.1365-2052.2003.01065.x. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J, Perez-Vizcaino F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells. Role of 5-HT2A receptors, caveolin-1, and Kv1.5 channel internalization. Circulation Research. 2006;98:860–862. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- Cool CD, Kennedy D, Voelkel NF, Tuder RM. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Human Pathology. 1997;28:434–442. doi: 10.1016/s0046-8177(97)90032-0. [DOI] [PubMed] [Google Scholar]
- Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of endothelial cell growth. The American Journal of Pathology. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool CD, Groshong SD, Oakey J, Voelkel NF. Pulmonary hypertension: cellular and molecular mechanisms. Chest. 2005;128:565–571. doi: 10.1378/chest.128.6_suppl.565S. [DOI] [PubMed] [Google Scholar]
- Cueva S, Sillau H, Valenzuela A, Ploog H. High altitude induced pulmonary hypertension and right ventricular failure in broiler chickens. Research in Veterinary Science. 1974;16:370–374. [PubMed] [Google Scholar]
- Dammann JF, McEachen JA, Thompson WM, Smith R, Muller WH. The regression of pulmonary vascular disease after the creation of pulmonary stenosis. The Journal of Thoracic and Cardiovascular Surgery. 1961;42:722–731. [PubMed] [Google Scholar]
- Deeb N, Shlosberg A, Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 4. Association between responses to heat stress and to cold-induced ascites. Poultry Science. 2002;81:1454–1462. doi: 10.1093/ps/81.10.1454. [DOI] [PubMed] [Google Scholar]
- De Jonghe F, Swinkels J. Selective serotonin reuptake inhibitors: relevance to differences in their pharmacologic and clinical profiles. Central Nervous System Drugs. 1997;7:452–467. [Google Scholar]
- Demiryurek AT, Wadsworth RM, Kane KA. Pharmacological evidence for the role of mediators in hypoxia-induced vasoconstriction in sheep isolated intrapulmonary artery rings. European Journal of Pharmacology. 1991;203:1–8. doi: 10.1016/0014-2999(91)90783-m. [DOI] [PubMed] [Google Scholar]
- Dorfmüller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, Capron F, Coulomb-Lherminé A, Marfaing-Koka A, Simonneau G, Emilie D, Humbert M. Chemokine RANTES in severe pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2002;165:534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- Dorfmüller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. European Respiratory Journal. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- Douglas JG, Munro JF, Kitchin AH, Muir AL, Proudfoot AT. Pulmonary hypertension and fenfluramine. British Medical Journal. 1981;283:881–883. doi: 10.1136/bmj.283.6296.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Sullivan CC, Chu D, Cho A, Kido JM, Wolfe PL, Yuan JX, Deutsch R, Jaimeson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. New England Journal of Medicine. 2003;348:500–509. doi: 10.1056/NEJMoa021650. [DOI] [PubMed] [Google Scholar]
- Duncker HR. Structure of avian lungs. Respiratory Physiology. 1972;14:44–63. doi: 10.1016/0034-5687(72)90016-3. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Adnot S. Anorexigen-induced pulmonary hypertension and the serotonin (5-HT) hypothesis: lessons for the future in pathogenesis. Respiratory Research. 2002;3:9–13. doi: 10.1186/rr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Adnot S. The serotonin pathway in p ulmonary hypertension. Advances in Pulmonary Hypertension. 2005;4(1):20–23. [Google Scholar]
- Eddahibi S, Raffestin B, Pham I, Launay JM, Aegerter P, Sitbon M, Adnot S. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. American Journal of Physiology. 1997;272:H1173–H1181. doi: 10.1152/ajpheart.1997.272.3.H1173. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Fabre V, Boni C, Martres MP, Raffestein B, Harmon M, Adnot S. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells - Relationship with the mitogenic action of serotonin. Circulation Research. 1999;84:329–336. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Hanoun N, Lanfumey L, Lesch K, Raffestin B, Harmon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transported gene. The Journal of Clinical Investigation. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Harmon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. The Journal of Clinical Investigation. 2001a;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Adnot S, Frisdale E, Levame M, Hamon M, Raffestin B. Dexfenfluramine-associated changes in 5-hydroxytryptamine transporter expression and development of hypoxic pulmonary hypertension in rats. The Journal of Pharmacology and Experimental Therapeutics. 2001b;297:148–154. [PubMed] [Google Scholar]
- Eddahibi S, Morrell N, d'Ortho MP, Naeije R, Adnot S. Pathobiology of pulmonary arterial hypertension. European Respiratory Journal. 2002;20:1559–1572. doi: 10.1183/09031936.02.00081302. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet A-S, Dartevelle P, Housset B, Hamon M, Weitzenblum E, Adnot S. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003;108:1839–1844. doi: 10.1161/01.CIR.0000091409.53101.E8. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Guignabert C, Barlier-Mur A-M, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension. Critical role for serotonin-induced smooth muscle hyperplasia. Circulation. 2006;113:1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- Egermayer P, Town GI, Peacock AJ. Role of serotonin in the pathogenesis of acute and chronic pulmonary hypertension. Thorax. 1999;54:161–168. doi: 10.1136/thx.54.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel G, Gothert MK, Hoyer D, Schlicker E, Hillebrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedeberg’s Archives of Pharmacology. 1986;357:1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- Enkvetchakul B, Beasley J, Bottje W. Pulmonary arteriole hypertrophy in broilers with pulmonary hypertension syndrome (ascites) Poultry Science. 1995;74:1676–1682. doi: 10.3382/ps.0741677. [DOI] [PubMed] [Google Scholar]
- Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. American Journal of Physiology. 1997;272:L795–L806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- Farber HW, Loscalzo J. Prothrombic hypertension mechanisms in primary pulmonary hypertension. The Journal of Laboratory and Clinical Medicine. 1999;134:561–566. doi: 10.1016/s0022-2143(99)90094-x. [DOI] [PubMed] [Google Scholar]
- Firth AL, Mandel J, Yuan JXJ. Idiopathic pulmonary arterial hypertension. Disease Models and Mechanisms. 2010;3:268–278. doi: 10.1242/dmm.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vessel media exhibit unique phenotypes and distinct growth capabilities. Circulation Research. 1997;81:940–952. doi: 10.1161/01.res.81.6.940. [DOI] [PubMed] [Google Scholar]
- Geiger R, Berger RM, Hess J, Bogers AJ, Sharma HS, Mooi WJ. Enhanced expression of vascular endothelial growth factor in pulmonary plexogenic arteriopathy due to congenital heart disease. The Journal of Pathology. 2000;191:202–207. doi: 10.1002/(SICI)1096-9896(200006)191:2<202::AID-PATH608>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Giaid A, Yanagisawa M, Langleben D, Michael RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Steward DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. New England Journal of Medicine. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. New England Journal of Medicine. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, Hamon M, Adnot S, Eddahibi S. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation. 2005;111:2812–2819. doi: 10.1161/CIRCULATIONAHA.104.524926. [DOI] [PubMed] [Google Scholar]
- Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur A-M, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circulation Research. 2006;98:1323–1330. doi: 10.1161/01.RES.0000222546.45372.a0. [DOI] [PubMed] [Google Scholar]
- Gust R, Schuster DP. Vascular remodeling in experimentally induced subacute canine pulmonary hypertension. Experimental Lung Research. 2001;27:1–12. doi: 10.1080/019021401459734. [DOI] [PubMed] [Google Scholar]
- Hamal KR, Wideman R, Anthony N, Erf GF. Expression of inducible nitric oxide synthase in lungs of broiler chickens following intravenous cellulose microparticle injection. Poultry Science. 2008;87:636–644. doi: 10.3382/ps.2007-00468. [DOI] [PubMed] [Google Scholar]
- Hamal KR, Wideman RF, Anthony NB, Erf GF. Differential gene expression of pro-inflammatory chemokines and cytokines in lungs of ascites-resistant and -susceptible broiler chickens following intravenous cellulose microparticle injection. Veterinary Immunology and Immunopathology. 2010a;133:250–255. doi: 10.1016/j.vetimm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Hamal KR, Wideman RF, Anthony NB, Erf GF. Differential expression of vasoactive mediators in the microparticle challenged lungs of chickens that differ in susceptibility to pulmonary arterial hypertension. American Journal of Physiology- Regulatory Integrative and Comparative Physiology. 2010b;298:R235–R242. doi: 10.1152/ajpregu.00451.2009. [DOI] [PubMed] [Google Scholar]
- Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease. A description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac defects. Circulation. 1958;18:533–547. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Repine JE. Platelets. In: Crystal RG, West JB, Barnes PJ, Weibel ER, editors. The Lung: Scientific Foundations. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997. pp. 947–959. [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hernandez A. Hypoxic ascites in broilers: A review of several studies done in Colombia. Avian Disease. 1987;31:171–183. [PubMed] [Google Scholar]
- Hervé P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. The American Journal of Medicine. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011–2020. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer FW, DeRuyck AMC. Pulmonary hypertension syndrome associated with ascites in broilers. The Veterinary Record. 1986;119:94. doi: 10.1136/vr.119.4.94. [DOI] [PubMed] [Google Scholar]
- Huchzermeyer FW, DeRuyck AMC, Van Ark H. Broiler pulmonary hypertension syndrome. III. Commercial broiler strains differ in their susceptibility. Onderstepoort Journal of Veterinary Research. 1988;55:5–9. [PubMed] [Google Scholar]
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Activation and pulmonary toxicity of pyrrolizidine alkaloids. Pharmacology & Therapeutics. 1990;47:371–389. doi: 10.1016/0163-7258(90)90063-8. [DOI] [PubMed] [Google Scholar]
- Hyvelin J-M, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circulation Research. 2005;97:185–191. doi: 10.1161/01.RES.0000174287.17953.83. [DOI] [PubMed] [Google Scholar]
- Ivy DD, McMurtry IF, Colvin K, Imamura M, Oka M, Lee D-S, Gebb S, James PL. Development of occlusive neointimal lesions in distal pulmonary arteries of endothelin B receptor-deficient rats: a new model of severe pulmonary artery hypertension. Circulation. 2005;111:2988–2996. doi: 10.1161/CIRCULATIONAHA.104.491456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SP, Greer JJM, Van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proceedings of the National academy of Sciences of the United States of America. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian RJ. Pulmonary hypertension as a cause of right ventricular failure and ascites in broilers. Zootecnica International. 1988 November #11;:58–62. [Google Scholar]
- Julian RJ. Lung volume of meat-type chickens. Avian Disease. 1989;33:174–176. [PubMed] [Google Scholar]
- Julian RJ. Ascites in poultry. Avian Pathology. 1993;22:419–454. doi: 10.1080/03079459308418934. [DOI] [PubMed] [Google Scholar]
- Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT(1B) receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT(1B)-receptor knockout mice and the 5-HT(1B/1D)-receptor antagonist GR127935. Circulation Research. 2001;89:1231–1239. doi: 10.1161/hh2401.100426. [DOI] [PubMed] [Google Scholar]
- Kelly JJ, Moore TM, Babal P, Diwan AH, Stephens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. American Journal of Physiology. 1998;274:L810–L819. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
- Kentera D, Susic D, Veljkovic V, Tukacovic G, Koko V. Pulmonary artery pressure in rats with hereditary platelet function defect. Respiration. 1988;54:110–114. doi: 10.1159/000195509. [DOI] [PubMed] [Google Scholar]
- Kuruma I, Okada T, Kataoka K, Sorimachi M. Ultrastructural observation of 5-hydroxytryptamine-storing granules in the domestic fowl thrombocytes. Zeitschrift Fuer Zellforschung Und Mikroskopische Anatomie. 1970;108:268–281. doi: 10.1007/BF00335298. [DOI] [PubMed] [Google Scholar]
- Lacoste-Eleaume AS, Bleux C, Quere P, Coudert F, Corbel C, Kanellopoulos-Langevin C. Biochemical and functional characterization of an avian homolog of the integrin GPIIb-IIIa present on chicken thrombocytes. Experimental Cell Research. 1994;213:198–209. doi: 10.1006/excr.1994.1191. [DOI] [PubMed] [Google Scholar]
- Lame MW, Jones AD, Wilson DW, Dunston SK, Segall HJ. Protein targets of monocrotaline pyrrole in pulmonary artery endothelial cells. The Journal of Biological Chemistry. 2000;275:29091–29099. doi: 10.1074/jbc.M001372200. [DOI] [PubMed] [Google Scholar]
- Launay JM, Hervé P, Peoc'h KK, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nature Medicine. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, Harmar AJ, Schmidt A-M, Lukanidin E, Rabinovitch M. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circulation Research. 2005;97:227–235. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- Le Cras TD, Kim DH, Gebb S, Markham NE, Shannon JM, Tuder RM, Abman SH. Abnormal lung growth and the development of pulmonary hypertension in the Fawn-Hooded rat. American Journal of Physiology. 1999;277:L709–L718. doi: 10.1152/ajplung.1999.277.4.L709. [DOI] [PubMed] [Google Scholar]
- Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture I. Characterization. American Journal of Physiology. 1986a;250:C761–C765. doi: 10.1152/ajpcell.1986.250.5.C761. [DOI] [PubMed] [Google Scholar]
- Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture II. stimulation by hypoxia. American Journal of Physiology. 1986b;250:C766–C770. doi: 10.1152/ajpcell.1986.250.5.C766. [DOI] [PubMed] [Google Scholar]
- Lee SL, Fanburg BL. Glycolytic activity and enhancement of serotonin uptake by endothelial cells exposed to hypoxia/anoxia. Circulation Research. 1987;60:653–658. doi: 10.1161/01.res.60.5.653. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circulation Research. 1991;68:1362–1368. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Lanzillo JJ, Fanburg BL. Serotonin produces both hyperplasia and hypertrophy of bovine pulmonary artery smooth muscle cells in culture. American Journal of Physiology. 1994;266:L46–L52. doi: 10.1152/ajplung.1994.266.1.L46. [DOI] [PubMed] [Google Scholar]
- Lee S-D, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. The Journal of Clinical Investigation. 1998;101:927–934. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase- induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circulation Research. 2004;95:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath R, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circulation Research. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- Lorenzoni AG, Anthony NB, BWideman RF. Transpulmonary pressure gradient verifies pulmonary hypertension is initiated by increased arterial resistance in broilers. Poultry Science. 2008;87:125–132. doi: 10.3382/ps.2007-00178. [DOI] [PubMed] [Google Scholar]
- Louis WJ. Primary pulmonary hypertension and anorectic drugs. New England Journal of Medicine. 1999;340:481–482. doi: 10.1056/NEJM199902113400614. [DOI] [PubMed] [Google Scholar]
- Loyd JE, Newman JH. Familial primary pulmonary hypertension. In: Rubin LJ, Rich S, editors. Primary Pulmonary Hypertension. New York, NY: Marcel Dekker, Inc.; 1997. pp. 151–162. [Google Scholar]
- Lubritz DL, Smith JL, McPherson BN. Heritability of ascites and the ratio of right to total ventricle weight in broiler breeder male lines. Poultry Science. 1995;74:1237–1241. doi: 10.3382/ps.0741237. [DOI] [PubMed] [Google Scholar]
- MacLean M, Sweeney RG, Baird M, McCulloch KM, Houslay M, Morecroft I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. British Journal of Pharmacology. 1996;119:917–930. doi: 10.1111/j.1476-5381.1996.tb15760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean M, Hervé P, Eddahibi S, Adnot S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. British Journal of Pharmacology. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina JN. Scanning electron microscope study of the spatial organization of air and blood conducting components of the avian lung (Gallus gallus variant domesticus) The Anatomical Record. 1988;222:145–153. doi: 10.1002/ar.1092220206. [DOI] [PubMed] [Google Scholar]
- Marcos E, Adnot S, Pham MH, Nosjean A, Raffestein B, Hamon M, Eddahibi S. Serotonin transporter inhibitors protect against hypoxic pulmonary vasoconstriction. American Journal of Respiratory and Critical Care Medicine. 2003;168:487–493. doi: 10.1164/rccm.200210-1212OC. [DOI] [PubMed] [Google Scholar]
- Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circulation Research. 2004;94:1263–1270. doi: 10.1161/01.RES.0000126847.27660.69. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Hester RK, Becker EJ, Jeffrey JS, Odom TW. Pulmonary artery endothelium-dependent vasodilation is impaired in a chicken model of pulmonary hypertension. American Journal of Physiology. 1999;277:R190–R197. doi: 10.1152/ajpregu.1999.277.1.R190. [DOI] [PubMed] [Google Scholar]
- Mason NA, Springall DR, Burke M, Pollack J, Mikhail G, Yacoub MH, Polak JM. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. The Journal of Pathology. 1998;185:313–318. doi: 10.1002/(SICI)1096-9896(199807)185:3<313::AID-PATH93>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Maxwell MH. Red cell size and various lung arterial measurements in different strains of domestic fowl. Research in Veterinary Science. 1991;50:233–239. doi: 10.1016/0034-5288(91)90113-3. [DOI] [PubMed] [Google Scholar]
- McGoon MD, Vanhoutte PM. Aggregating platelets contract isolated canine pulmonary arteries by releasing 5-hydroxytryptamine. The Journal of Clinical Investigation. 1984;74:828–833. doi: 10.1172/JCI111499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin VV. Pulmonary arterial hypertension: current diagnosis and management. American College of Cardiology Current Journal Review. 2002;11(3):17–21. [Google Scholar]
- Mecham RP, Whitehouse LA, Wrenn DS, Parks WC, Griffin GL, Senior RM, Crouch EC, Stenmark KR, Voelkel NF. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science. 1987;237:423–426. doi: 10.1126/science.3603030. [DOI] [PubMed] [Google Scholar]
- Medhora M, Bousamra II M, Zhu D, Somberg L, Jacobs ER. Upregulation of collagens detected by gene array in a model of flow-induced pulmonary vascular remodeling. American Journal of Physiology- Heart and Circulation Physiology. 2002;282:H414–H422. doi: 10.1152/ajpheart.00292.2001. [DOI] [PubMed] [Google Scholar]
- Moghadam HK, McMillan I, Chambers JR, Julian RJ. Estimation of genetic parameters for ascites syndrome in broiler chickens. Poultry Science. 2001;80:844–848. doi: 10.1093/ps/80.7.844. [DOI] [PubMed] [Google Scholar]
- Morecroft I, Heeley RP, Prentice HM, Kirk A, MacLean MR. 5-HT receptors mediating contraction in human small pulmonary arteries: importance of the 5-HT1B receptor. British Journal of Pharmacology. 1999;128:730–734. doi: 10.1038/sj.bjp.0702841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno de Sandino M, Hernandez A. Nitric oxide synthase expression in the endothelium of pulmonary arterioles in normal and pulmonary hypertensive chickens subjected to chronic hypobaric hypoxia. Avian Disease. 2003;47:1291–1297. doi: 10.1637/6006. [DOI] [PubMed] [Google Scholar]
- Moreno de Sandino M, Hernandez A. Pulmonary arteriole remodeling in hypoxic broilers expressing different amounts of endothelial nitric oxide synthase. Poultry Science. 2006;85:899–901. doi: 10.1093/ps/85.5.899. [DOI] [PubMed] [Google Scholar]
- Morse JH. Defining the role and clinical relevance of BMPR2 mutations in pulmonary arterial hypertension. Advances in Pulmonary Hypertension. 2005;4(1):5–12. [Google Scholar]
- Naeije R, Eddahibi S. Serotonin in pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2004;70:209–210. doi: 10.1164/rccm.2406002. [DOI] [PubMed] [Google Scholar]
- Naeye RL, Vennart GP. The structure and significance of pulmonary plexiform structures. The American Journal of Pathology. 1959;36:593–605. [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. Journal of Applied Physiology. 2006;100:996–1002. doi: 10.1152/japplphysiol.01028.2005. [DOI] [PubMed] [Google Scholar]
- Nauser TD, Stites SW. Diagnosis and treatment of pulmonary hypertension. American Family Physician. 2001;63(9):1789–1799. [PubMed] [Google Scholar]
- Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, Coccolo F, Ventura C, Phillips JA, Knowles JA, Janssen B, Eickelberg O, Eddahibi S, Herve P, Nichols WC, Elliot G. Genetic basis of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2004;43:33S–39S. doi: 10.1016/j.jacc.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Nicolls MR, Taraseviciene-Stewart T, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. European Respiratory Journal. 2005;26:1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qui D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. American Journal of Respiratory and Critical Care Medicine. 2002;166:1403–1408. doi: 10.1164/rccm.200203-268OC. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GM, Shi L, Qui D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- Owen RL, Wideman RF, Jr, Hattel AL, Cowen BS. Use of a hypobaric chamber as a model system for investigating ascites in broilers. Avian Disease. 1990;34:754–758. [PubMed] [Google Scholar]
- Owen RL, Wideman RF, Leach RM, Cowen BS, Dunn PA, Ford BC. Physiologic and electrocardiographic changes occurring in broilers reared at simulated high altitude. Avian Disease. 1995a;39:108–115. [PubMed] [Google Scholar]
- Owen RL, Wideman RF, Barbato GF, Cowen BS, Ford BC, Hattel AL. Morphometric and histologic changes in the pulmonary system of broilers raised at simulated high altitude. Avian Pathology. 1995b;24:293–302. doi: 10.1080/03079459508419070. [DOI] [PubMed] [Google Scholar]
- Owen RL, Wideman RF, Jr, Cowen BS. Changes in pulmonary arterial and femoral arterial pressure upon acute exposure to hypobaric hypoxia in broiler chickens. Poultry Science. 1995c;74:708–715. doi: 10.3382/ps.0740708. [DOI] [PubMed] [Google Scholar]
- Pakala R, Willerson JT, Benedict CR. Mitogenic effect of serotonin on vascular endothelial cells. Circulation. 1994;90:1919–1926. doi: 10.1161/01.cir.90.4.1919. [DOI] [PubMed] [Google Scholar]
- Pakdel A, Van Arendonk JAM, Vereijken ALJ, Bovenhuis H. Direct and maternal genetic effects for ascites-related traits in broilers. Poultry Science. 2002;81:1273–1279. doi: 10.1093/ps/81.9.1273. [DOI] [PubMed] [Google Scholar]
- Palevsky HI, Schloo BL, Pietra GG, Weber KT, Janicki JS, Ruben E, Fishman AP. Primary pulmonary hypertension: vascular structure, morphometry and responsiveness to vasodilator agents. Circulation. 1989;80:1207–1221. doi: 10.1161/01.cir.80.5.1207. [DOI] [PubMed] [Google Scholar]
- Pan J-Q, Tan X, Li J-C, Sun W-D, Wang X-L. Effects of early feed restriction and cold temperature on lipid peroxidation, pulmonary vascular remodeling and ascites morbidity in broilers under normal and cold temperatures. British Poultry Science. 2005;46:374–381. doi: 10.1080/00071660500098152. [DOI] [PubMed] [Google Scholar]
- Peacock AJ, Pickett C, Morris K, Reeves JT. The relationship between rapid growth and pulmonary hemodynamics in the fast-growing broiler chicken. American Review of Respiratory Disease. 1989;139:1524–1530. doi: 10.1164/ajrccm/139.6.1524. [DOI] [PubMed] [Google Scholar]
- Peacock AJ, Pickett C, Morris K, Reeves JT. Spontaneous hypoxaemia and right ventricular hypertrophy in fast growing broiler chickens reared at sea level. Comparative Biochemistry and Physiology. 1990;97A:537–541. doi: 10.1016/0300-9629(90)90124-b. [DOI] [PubMed] [Google Scholar]
- Pietra GG. The pathology of primary pulmonary hypertension. In: Rubin LJ, Rich S, editors. Primary Pulmonary Hypertension: Lung Biology in Health and Disease. New York, NY: Marcel Dekker, Inc.; 1997. pp. 19–61. [Google Scholar]
- Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. Journal of the American College of Cardiology. 2004;43:25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Pitt BR, Weng W, Steve AR, Blakely RD, Reynolds I, Davies P. Serotonin increases DNA synthesis in rat proximal and distal pulmonary vascular smooth muscle cells in culture. American Journal of Physiology. 1994;266:L178–L186. doi: 10.1152/ajplung.1994.266.2.L178. [DOI] [PubMed] [Google Scholar]
- Ploog HP. M.S. Thesis. University Park, PA: The Pennsylvania State University; 1973. Physiologic changes in broiler chickens (Gallus domesticus) exposed to a simulated altitude of 4267 m (14000 ft) [Google Scholar]
- Rabinovitch M. Insights into the pathogenesis of primary pulmonary hypertension from animal models. In: Rubin LJ, Rich S, editors. Primary Pulmonary Hypertension. New York, NY: Marcel Dekker, Inc.; 1997. pp. 63–82. [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. Clinical insights into the pathogenesis of primary pulmonary hypertension. Chest. 1998;114:237S–241S. doi: 10.1378/chest.114.3_supplement.237s. [DOI] [PubMed] [Google Scholar]
- Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor- β signaling in idiopathic pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2004;170:1340–1348. doi: 10.1164/rccm.200311-1602OC. [DOI] [PubMed] [Google Scholar]
- Rodat-Despoix L, Crevel H, Marthan R, Savineau J0P, Guibert C. Heterogeneity in 5HT-induced contractile and proliferative responses in rat pulmonary arterial bed. Journal of Vascular Research. 2008;45:181–192. doi: 10.1159/000111071. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Ayestas MA, Dersch CM, Bauman MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation. 1999;100:869–875. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- Rubin LJ. Primary pulmonary hypertension. New England Journal of Medicine. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- Russell BR, Laverty R. Correlation between 5-HT content and uptake site density following (S)-MDMA and dexfenfluramine-induced depletion, and with neuroprotection by the glycine site-specific NMDA antagonist ACEA 1021. Annals of the New York Academy of Sciences. 2000;914:208–214. doi: 10.1111/j.1749-6632.2000.tb05197.x. [DOI] [PubMed] [Google Scholar]
- Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. The Journal of the Federation of American Societies for Experimental Biology. 2005;19:1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- Saldana ME, Harley RA, Liebow AA, Carrington CB. Experimental extreme pulmonary hypertension and vascular disease in relation to polycythemia. The American Journal of Pathology. 1968;52:935–981. [PMC free article] [PubMed] [Google Scholar]
- Sato K, Webb S, Tucker A, Rabinovitch M, O’Brien RF, McMurtry IF, Stelzner TJ. Factors influencing the idiopathic development of pulmonary hypertension in the fawn hooded rat. American Review of Respiratory Disease. 1992;145:793–797. doi: 10.1164/ajrccm/145.4_Pt_1.793. [DOI] [PubMed] [Google Scholar]
- Schnader SM, Schloo BL, Anderson W, Stephenson LW, Fishman AP. Chronic pulmonary hypertension in sheep: temporal progression of lesions. Journal of Surgical Research. 1996;62:243–250. doi: 10.1006/jsre.1996.0202. [DOI] [PubMed] [Google Scholar]
- Seiler KU, Wassermann O, Wensky H. On the role of serotonin in the pathogenesis of pulmonary hypertension induced by anorectic drugs, an experimental study in the isolated perfused rat lung. Clinical and Experimental Pharmacology and Physiology. 1974;1:463–471. doi: 10.1111/j.1440-1681.1976.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Sibbald W, Peters S, Lindsay RM. Serotonin and pulmonary hypertension in human septic ARDS. Critical Care Medicine. 1980;8:490–494. doi: 10.1097/00003246-198009000-00003. [DOI] [PubMed] [Google Scholar]
- Sillau AH, Montalvo C. Pulmonary hypertension and the smooth muscle of pulmonary arterioles in chickens at high altitude. Comparative Biochemistry and Physiology. 1982;71A:125–130. [Google Scholar]
- Silversides FG, LeFrancois MR, Villeneuve P. The effect of strain of broiler on physiological parameters associated with the ascites syndrome. Poultry Science. 1997;76:663–667. doi: 10.1093/ps/76.5.663. [DOI] [PubMed] [Google Scholar]
- Simoneit LW, Belamarich FA, Shepro D. The localization of 5-hydroxytryptamine in blood cells in nonmammalian vertebrates. The Journal of Cell Biology. 1970;47:192–193. [Google Scholar]
- Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Sorimachi M, Kataoka K, Inouye A. Uptake of 5-hydroxytryptamine by domestic fowl thrombocytes (spindle cells) in vitro. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1974;283:53–65. doi: 10.1007/BF00500145. [DOI] [PubMed] [Google Scholar]
- Sorimachi M, Kataoka K, Inouye A, Hori S. Release in vitro of 5-hydroxytryptamine from spindle cells of the domestic fowl. European Journal of Pharmacology. 1970;10:243–248. doi: 10.1016/0014-2999(70)90279-7. [DOI] [PubMed] [Google Scholar]
- Stelzner TJ, O’Brien RF, Sato K, Webb S, Zamora MR, Sakurai T, Yanagisawa M, McMurtry IF, Fisher J. Increased endothelin-1 expression in rats with pulmonary hypertension. American Journal of Physiology. 1992;262:L614–L620. doi: 10.1152/ajplung.1992.262.5.L614. [DOI] [PubMed] [Google Scholar]
- Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. American Journal of Physiology- Lung Cellular and Molecular Physiology. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- Stevens T. Molecular and cellular determinants of lung endothelial cell heterogeneity. Chest. 2005;128:558–564. doi: 10.1378/chest.128.6_suppl.558S. [DOI] [PubMed] [Google Scholar]
- Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FJ, Garcia JGN, Hebbel RP, Tuder RM, Garfinkel S. NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. American Journal of Physiology- Cell Physiology. 2001;281:C1422–C1433. doi: 10.1152/ajpcell.2001.281.5.C1422. [DOI] [PubMed] [Google Scholar]
- Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12331–12336. doi: 10.1073/pnas.1933740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Liu Y-J, Li J-C, Pan J-Q, Sun W-D, Wang X-L. Activation of PCKα and pulmonary vascular remodeling in broilers. Research in Veterinary Science. 2005a;79:131–137. doi: 10.1016/j.rvsc.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Tan X, Pan J-Q, Li J-C, Liu Y-J, Sun W-D, Wang X-L. L-Arginine inhibiting pulmonary vascular remodelling is associated with promotion of apoptosis in pulmonary arterioles smooth muscle cells in broilers. Research in Veterinary Science. 2005b;79:203–209. doi: 10.1016/j.rvsc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Schuster DP, Davis EC, Patterson GA, Botney MD. The role of vascular injury and hemodynamics in rat pulmonary artery remodeling. The Journal of Clinical Investigation. 1996;98:434–442. doi: 10.1172/JCI118809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. The Journal of the Federation of American Societies for Experimental Biology. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- Taraseviciene-Stewart L, Scerbavicius R, Choe K-H, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. American Journal of Physiology- Lung Cellular and Molecular Physiology. 2006;291:L668–L676. doi: 10.1152/ajplung.00491.2005. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. The American Journal of Pathology. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- Tuder RM, Lee S-D, Cool CC. Histopathology of pulmonary hypertension. Chest. 1998a;114:1S–6S. doi: 10.1378/chest.114.1_supplement.1s-a. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Radisavljevic Z, Shroyer KR, Polak JM, Voelkel NF. Monoclonal endothelial cells in appetite suppressant-associated pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 1998b;158:1999–2001. doi: 10.1164/ajrccm.158.6.9805002. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch DB, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza L, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. The Journal of Pathology. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- Tyler RC, Muramatsu M, Abman SH, Selzner TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. American Journal of Physiology. 1999;276:L297–L303. doi: 10.1152/ajplung.1999.276.2.L297. [DOI] [PubMed] [Google Scholar]
- Uzun O, Demiryurek AT, Kanzik I. The role of tyrosine kinase in hypoxic constriction of sheep pulmonary artery rings. European Journal of Pharmacology. 1998;358:41–47. doi: 10.1016/s0014-2999(98)00592-5. [DOI] [PubMed] [Google Scholar]
- Vaszar LT, Nishimura T, Storey JD, Zhao G, Qui D, Faul JL, Pearl RG, Kao PN. Longitudinal transcriptional analysis of developing neointimal vascular occlusion and pulmonary hypertension in rats. Physiological Genomics. 2004;17:150–156. doi: 10.1152/physiolgenomics.00198.2003. [DOI] [PubMed] [Google Scholar]
- Villamor E, Ruijtenbeek K, Pulgar V, De Mey JGR, Blanco CE. Vascular reactivity in intrapulmonary arteries of chicken embryos during transition to ex ovo life. American Journal of Physiology. 2002;282:R917–R927. doi: 10.1152/ajpregu.00369.2001. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Tuder RM, Weir EK. Pathophysiology of primary pulmonary hypertension: from physiology to molecular mechanisms. In: Rubin LJ, Rich S, editors. Primary Pulmonary Hypertension. New York, NY: Marcel Dekker, Inc.; 1997. pp. 83–129. [Google Scholar]
- Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1998;114:225S–230S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension: a pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation. 1970;42:1163–1184. [Google Scholar]
- Wang W, Wideman RF, Bersi TK, Erf GF. Pulmonary and hematological inflammatory responses to intravenous cellulose micro-particles in broilers. Poultry Science. 2003;82:771–780. doi: 10.1093/ps/82.5.771. [DOI] [PubMed] [Google Scholar]
- Weir EK, Reeve HL, Huang JM, Michelakis E, Nelson DP, Hampl V, Archer SL. Anorexic agents aminorex, fenfluramine, and dexfenfluramine inhibit potassium current in rat pulmonary vascular smooth muscle and cause pulmonary vasoconstriction. Circulation. 1996;94:2216–2220. doi: 10.1161/01.cir.94.9.2216. [DOI] [PubMed] [Google Scholar]
- Wideman RF. Cardiac Output in four-, five- and six-week-old broilers, and hemodynamic responses to intravenous injections of epinephrine. Poultry Science. 1999;78:392–403. doi: 10.1093/ps/78.3.392. [DOI] [PubMed] [Google Scholar]
- Wideman RF. Cardio-pulmonary hemodynamics and ascites in broiler chickens. Avian and Poultry Biology Reviews. 2000;11(1):21–43. [Google Scholar]
- Wideman RF. Pathophysiology of heart/lung disorders: pulmonary hypertension syndrome in broiler chickens. World’s Poultry Science Journal. 2001;57:289–307. [Google Scholar]
- Wideman RF, Kirby YK. A pulmonary artery clamp model for inducing pulmonary hypertension syndrome (ascites) in broilers. Poultry Science. 1995a;74:805–812. doi: 10.3382/ps.0740805. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Kirby YK. Evidence of a ventilation-perfusion mismatch during acute unilateral pulmonary artery occlusion in broilers. Poultry Science. 1995b;74:1209–1217. doi: 10.3382/ps.0741209. [DOI] [PubMed] [Google Scholar]
- Wideman RF, French H. Broiler breeder survivors of chronic unilateral pulmonary artery occlusion produce progeny resistant to pulmonary hypertension syndrome (ascites) induced by cool temperatures. Poultry Science. 1999;78:404–411. doi: 10.1093/ps/78.3.404. [DOI] [PubMed] [Google Scholar]
- Wideman RF, French H. Ascites resistance of progeny from broiler breeders selected for two generations using chronic unilateral pulmonary artery occlusion. Poultry Science. 2000;79:396–401. doi: 10.1093/ps/79.3.396. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Erf GF. Intravenous micro-particle injections trigger pulmonary hypertension in broiler chickens. Poultry Science. 2002;81:877–886. doi: 10.1093/ps/81.6.877. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Kirby YK, Tackett CD, Marson NE, McNew RW. Cardio-pulmonary function during acute unilateral occlusion of the pulmonary artery in broilers fed diets containing normal or high levels of arginine-HCl. Poultry Science. 1996a;75:1587–1602. doi: 10.3382/ps.0751587. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Kirby YK, Tackett CD, Marson NE, Tressler CJ, McNew RW. Independent and simultaneous unilateral occlusion of the pulmonary artery and extra-pulmonary primary bronchus in broilers. Poultry Science. 1996b;75:1417–1427. doi: 10.3382/ps.0751417. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Kirby YK, Owen RL, French H. Chronic unilateral occlusion of an extra-pulmonary primary bronchus induces pulmonary hypertension syndrome (ascites) in male and female broilers. Poultry Science. 1997;76:400–404. doi: 10.1093/ps/76.2.400. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Fedde MR, Tackett CD, Weigle GE. Cardio-pulmonary function in preascitic (hypoxemic) or normal broilers inhaling ambient air or 100% oxygen. Poultry Science. 2000;79:415–425. doi: 10.1093/ps/79.3.415. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Erf GF, Chapman ME, Wang W, Anthony NB, Xiaofong L. intravenous micro-particle injections and pulmonary hypertension in broiler chickens: Acute post-injection mortality and ascites susceptibility. Poultry Science. 2002;81:1203–1217. doi: 10.1093/ps/81.8.1203. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Chapman ME, Owens CM, Devabhaktuni MK, Cavitt LC, Erf GF. Broiler survivors of intravenous micro-particle injections: Evaluation of growth, livability, meat quality, and arterial blood gas values during a cyclic heat challenge. Poultry Science. 2003;82:484–495. doi: 10.1093/ps/82.3.484. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Chapman ME, Wang W, Erf GF. Immune modulation of the pulmonary hypertensive response to bacterial lipopolysaccharide (endotoxin) in broilers. Poultry Science. 2004;83:624–637. doi: 10.1093/ps/83.4.624. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Erf GF, Chapman ME. Nω-Nitro-L-Arginine Methyl Ester (L-NAME) amplifies the pulmonary hypertensive response to micro-particle injections in broilers. Poultry Science. 2005;84:1077–1091. doi: 10.1093/ps/84.7.1077. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Bowen OT, Erf GF, Chapman ME. Influence of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on the pulmonary hypertensive response to micro-particle injections in broilers. Poultry Science. 2006;85:511–527. doi: 10.1093/ps/85.3.511. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Chapman ME, Hamal KR, Bowen OT, Lorenzoni AG, Erf GF, Anthony NB. An inadequate pulmonary vascular capacity and susceptibility to pulmonary arterial hypertension in broilers. Poultry Science. 2007;86:984–998. doi: 10.1093/ps/86.5.984. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Eanes ML, Hamal KR, Anthony NB. Pulmonary vascular pressure profiles in broilers selected for susceptibility to pulmonary hypertension syndrome: Age and gender comparisons. Poult Sci. 2010a;89:1815–1824. doi: 10.3382/ps.2010-00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman RF, Hamal KR, Bayona MT, Lorenzoni AG, Cross D, Khajali F, Rhoads DD, Erf GF, Anthony NB. Plexiform lesions in the lungs of domestic fowl selected for susceptibility to pulmonary arterial hypertension: Incidence and histology. The Anatomical Record. 2010b doi: 10.1002/ar.21369. (in press) [DOI] [PubMed] [Google Scholar]
- Wright L, Tuder RM, Wang J, Cool CD, Lepley RA, Voelkel NF. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 1998;157:219–229. doi: 10.1164/ajrccm.157.1.9704003. [DOI] [PubMed] [Google Scholar]
- Xiang RP, Sun WD, Wang JY, Wang XL. Effect of vitamin C on pulmonary hypertension and muscularization of pulmonary arterioles in broilers. British Poultry Science. 2002;43:705–712. doi: 10.1080/0007166021000025064. [DOI] [PubMed] [Google Scholar]
- Xiang RP, Sun WD, Zhang KC, Li JC, Wang JY, Wang XL. Sodium chloride-induced acute and chronic pulmonary hypertension syndrome in broiler chickens. Poultry Science. 2004;83:732–736. doi: 10.1093/ps/83.5.732. [DOI] [PubMed] [Google Scholar]
- Yamaki S, Wagenvoort CA. Comparison of primary plexogenic arteriopathy in adults and children. British Heart Journal. 1985;54:428–434. doi: 10.1136/hrt.54.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2000;162:1577–1586. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- Zabka TS, Campbell FE, Wilson DW. Pulmonary arteriopathy and idiopathic pulmonary arterial hypertension in six dogs. Veterinary Pathology. 2006;43:510–522. doi: 10.1354/vp.43-4-510. [DOI] [PubMed] [Google Scholar]
- Zamora MR, Stelzner TJ, Webb S, Panos RJ, Ruff LJ, Dempsey EC. Overexpression of endothelin-1 and enhanced growth of pulmonary artery smooth muscle cells from fawn-hooded rats. American Journal of Physiology. 1996;270:L101–L109. doi: 10.1152/ajplung.1996.270.1.L101. [DOI] [PubMed] [Google Scholar]