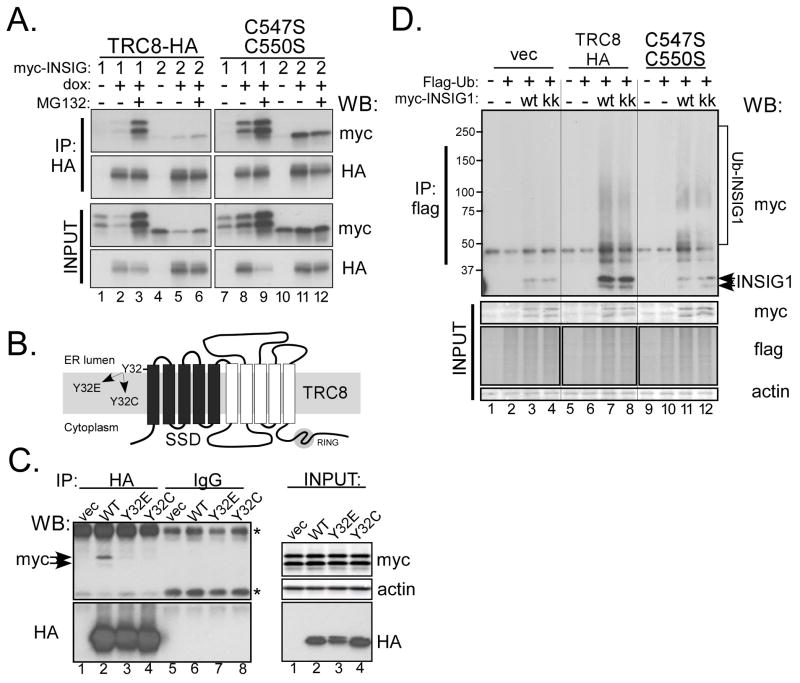
Figure 3. TRC8 binds and ubiquitylates INSIG.
(A) TRC8-HA-FlpIn cells (wt or C547S;C550S RING mutant), cultured in sterol-replete DMEM (10% FCS), were transfected overnight with 300ng of pCMV-INSIG-1-myc or pCMV-INSIG-2-myc, then dox-treated or not for 24 h. Where indicated, MG132 was added 4h prior to harvest. CHAPSO lysates (500μg) were immunoprecipitated with anti-HA and pellets analyzed by Western blot for INSIG (myc) and TRC8-HA (HA). (B) Diagram of TRC8 showing 10 TM segments (bars), the first five of which comprise the SSD (black bars). TRC8 also contains a RING-H2 domain in the C-terminus (shaded circle). The conserved tyrosine 32, in the first TM of the SSD, was mutated to glutamate or cysteine and used for the IP experiments in panel C. (C) Control HEK293 cells (vec) and stable transfectants expressing wild type or SSD mutant TRC8 (Y32E or Y32C) were transfected with INSIG-1-myc and harvested after 48h. TritonX-100 lysates containing 400ug of protein were immunoprecipitated using anti-HA or control beads. IP pellets were analyzed for co-precipitated INSIG-1 (myc) and TRC8 (HA). Input blots verified equal expression; asterisks indicate background IgG bands. (D) Control cells (vec) and stable transfectants expressing wild type or RING mutant TRC8 (C547S, C550S) were co-transfected with flag-ubiquitin and myc-INSIG-1 (wt) or the K156R, K158R mutant (kk), as indicated. Cells were dox-induced for 24 h and treated with MG132 for 2h prior to harvest. TritonX-100 lysates (200 μg aliquots) were immunoprecipitated with anti-flag beads and analyzed on Western blots with the indicated antibodies. Input blots verified equal expression; conjugated ubiquitin, detected with anti-flag antibodies, was used to verify equal expression because unconjugated ubiquitin was not visible.