Abstract
OBJECTIVES
To determine whether the presence of high depressive symptoms diminished physical performance benefits after a comprehensive physical activity intervention in older adults.
STUDY DESIGN
A post-hoc analysis of data from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study which was a single blind randomized controlled trial comparing a moderate intensity physical activity intervention (PA) with a successful aging control (SA).
SETTING
Multi-center U.S. sites participating in the LIFE-P trial.
PARTICIPANTS
LIFE-P trial participants included 424 sedentary, non-institutionalized adults (70–89 years).
MEASUREMENTS
Depressive symptoms were assessed by the Centers for Epidemiological Studies Depression Scale (CES-D). Physical performance tests included the Short Physical Performance Battery (SPPB) and 400 meter walk time (400 mw) at baseline, 6 and 12 months.
RESULTS
Of the participants, 15.8% had high depressive symptom scores (CES-D ≥ 14). For participants with low depressive symptoms, SPPB scores improved in the PA versus the SA group over 12 months (adjusted score difference: +0.70; p = <0.001 at 6 months and +0.58; p=0.004 at 12 months) while the 400 mw times improved in the PA group at 6 months (adjusted score difference −0.41 min.; p=0.021). For those with high depressive symptoms, a trend toward statistical improvement in the SPPB was observed in the PA versus SA group (adjusted score difference +0.76 (p=0.176) at 6 months and +0.94 (p=0.116) at 12 months).
CONCLUSION
The presence of high depressive symptoms did not substantially diminish physical performance benefits realized after a PA intervention in sedentary older adults.
INTRODUCTION
Depressive symptoms are associated with decline in physical performance in older adults in both cross-sectional and longitudinal epidemiological studies (1–3). A diagnosis of depression has been associated with low physical activity levels in older adults (4, 5) and is considered a risk factor for mobility disability (6). The presence of elevated or high depressive symptoms in the absence of a clinical diagnosis of depression may be associated with the same negative effects on functional ability and health outcomes found in those with a diagnosis of major depression (7). Sedentary older adults, especially those with symptoms of depression, would appear logical target groups for exercise interventions that improve physical performance (8, 9). However, poor adherence rates and self-efficacy in older adults with depression have been reported as potential barriers to the success of exercise interventions (6,10). Whether the presence of high depressive symptoms, independent of such barriers, modifies physical performance benefits resulting from structured exercise interventions is unclear.
A relationship between increasing levels of physical activity and decreasing depressive symptoms has been demonstrated independent of physical and psychological health (11, 12). Multiple clinical trials have demonstrated that physical activity may be an effective strategy to reduce depressive symptoms in older adults (13–16). The potential anti-depressive benefits of physical activity further complicate the ability to understand how depressive symptoms influence physical performance outcomes after an exercise intervention. Reduction in depression scores, concomitant with improvements in performance, has largely been demonstrated in exercise trials that have targeted clinically depressed individuals (13, 14, 17). It is less clear whether exercise improves mood in non-depressed individuals (15). In addition, the mechanistic relationship between physical activity and depression is not well understood but may vary by exercise mode, intensity, duration, and baseline depression level (17).
Recent findings from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study found significant improvements in lower extremity physical performance for participants in a physical activity (PA) intervention compared to a successful aging (SA) control (18). Understanding whether the presence of co-morbid factors such as high depressive symptoms influence outcomes from an exercise intervention is relevant to identifying appropriate target groups. Using data from the LIFE-P study (18), the present study examined whether benefits in physical performance outcomes are modified by the presence of high depressive symptoms after implementation of a PA intervention. It was hypothesized that the positive effect of the PA intervention on physical performance would be diminished in those with high depressive symptoms scores. A secondary hypothesis that depression scores would improve in those with high depressive symptoms after a PA intervention is also explored.
METHODS
Study Design
This study is a secondary analysis of data from the LIFE-P trial previously described by investigators (19). Briefly, LIFE-P was a single-blind, multi-center, randomized controlled study contrasting a PA intervention with a SA education control in sedentary older adults, with physical performance outcome measures including the SPPB and 400 mw time proximal to the primary outcome measure of major mobility disability. Here data were analyzed to examine whether the baseline depressive symptom scores modify physical performance outcomes.
Participants
Inclusion and exclusion criteria and recruitment are described elsewhere (18, 19). Participants were 424 older adults at risk for lower extremity disability due to low physical activity levels. Eligibility criteria were: (a) age 70–89 years, (b) sedentary lifestyle (<20 min per week spent in structured PA during the past month), (c) ability to walk 400 meters within 15 minutes without sitting or use of an assistive device, and (d) having an SPPB score < 10 (18, 19).
Interventions
The PA intervention utilized a combination of aerobic, strength, flexibility and balance exercises along three phases. The adoption phase (weeks 1–8) involved an introductory individualized session followed by three 40–60 minute center-based group sessions per week. In the transition phase (weeks 9–24) two center-based exercise sessions per week were combined with home-based endurance/strength/flexibility exercises at least three times per week. The maintenance phase (weeks 25 to end of trial) utilized a home-based intervention along with group-based behavioral counseling sessions (1 time per week for the first 10 weeks), walking (goal of a minimum of 150 minutes per week) and optional one or two group sessions per week.
The SA control participants met in small groups weekly for the first 26 weeks, monthly thereafter, and were given a series of sessions on health topics relevant to older adults. At the end of each session, 5 to 10 minutes of upper extremity stretching exercises were provided.
Measurements
Demographic and clinical variables
Baseline interviews included anthropometric measures, a physical exam, electrocardiogram, a physician evaluation, and a Mini-Mental Status Examination (MMSE) (20). Clinical conditions and medications were assessed using self-reported, physician-diagnosed disease information. PA was self-reported using the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (21).
Depression
Depressive symptoms were assessed using the 20 item Center for Epidemiological Studies Depression (CES-D) scale which measures depressive feelings and behaviors experienced in the past week (22). On this scale (0 to 60), a score of 16 or greater indicates depression. To improve power in this analysis, participants were separated into groups of “low” and “high” depressive symptoms utilizing one standard deviation above the mean scoring. This resulted in a score of 14 or greater representing those with “high” depressive symptoms versus a score of less than 14 denoting “low” depressive symptoms.
Physical performance measures
The Short Physical Performance Battery (SPPB) includes a 4 meter self-paced walking speed, balance tests, and chair stand tests. A categorical score in each of the three areas (0–4), and a summary score is determined (0–12) with a higher score indicating better performance (23). A 400 meter walk time (400 mw) involved participants walking 10 laps of a 40-meter course at a comfortable, self-directed pace (19). Time to complete the course was recorded in minutes and seconds.
Statistical Analysis
Statistical analyses were performed using SAS software, (SAS Version 9.1, SAS Institute, Inc. Cary, NC).
Sample means and standard deviations were computed for the continuous descriptive characteristics, and the count and proportions were calculated for the discrete descriptive characteristics, within intervention groups and depressive symptom groups. To minimize the heterogeneity of variance, variables were transformed to best approximate the conditional normality assumption if necessary. Comparisons of continuous variables were performed using the Kruskal-Wallis test if not normally distributed and analysis of variance (ANOVA) if normally distributed and comparisons of discrete baseline characteristics were performed using chi-square tests.
Differences in mean SPPB and 400 mw time between intervention groups were estimated using repeated measures analysis of covariance (ANCOVA). The baseline measures, age, gender (stratifying variables for randomization), clinic site, intervention assignment, visit interval, and an intervention by visit interaction were included in the model by depressive symptom groups. Tests for intervention effects at the 6 and 12 month assessment visits were performed using contrasts of the 6 and 12 month intervention means. Overall comparisons between groups for SPPB and 400 mw across follow-up visits were obtained using a contrast to compare average effects across both follow-up visits. The interaction between depressive symptom groups and intervention was also examined. Differences in CES-D score between intervention groups were estimated using repeated measures ANCOVA with the baseline CES-D, age, gender, clinic site, intervention assignment, visit, and an intervention by visit interaction included in the model.
RESULTS
Table 1 shows the baseline characteristics of the LIFE-P study participants stratified by depressive symptoms and intervention arm. Mean age of participants was 76.77 (+/− 4.24 years). Demographic, medical, and anthropometric characteristics did not significantly differ in the PA and SA groups. The prevalence of those with high depressive symptoms at baseline was 17.8% in the PA intervention and 13.7% in the SA control arm resulting in no statistically significant differences in baseline characteristics in general. A slightly higher prevalence of myocardial infarction, diabetes, cancer, hypertension, and congestive heart failure was seen for participants with high versus low depressive symptoms in both study arms. The number of participants taking antidepressant medication was significantly higher in those with high depressive versus low symptoms but comparable between the two intervention arms. The total number of prescription drugs taken was also higher for those with high depressive symptoms. Cognitive scores did not statistically differ for those with high versus low depressive symptoms.
Table 1.
Baseline Characteristics of All Participants by Treatment Arm and Depressive Symptoms*
| Physical Activity Intervention | Successful Aging Control | |||
|---|---|---|---|---|
| High Depressive Symptoms |
Low Depressive Symptoms |
High Depressive Symptoms |
Low Depressive Symptoms |
|
| Age (years) | 75.74±4.71 (N=38) | 76.70±4.04 (N=175) | 75.24±3.64 (N=29) | 77.29±4.35 (N=182) |
| Race/Ethnicity | ||||
| African American/Black | 8/38 (21.1%) | 29/175 (16.6%) | 6/29 (20.7%) | 34/182 (18.7%) |
| Caucasian/White | 25/38 (65.8%) | 135/175 (77.1%) | 20/29 (69.0%) | 135/182 (74.2%) |
| Other/Mixed | 5/38 (13.6%) | 11/175 (6.3%) | 3/29 (10.3%) | 13/182 (7.1%) |
| Gender | ||||
| Female | 24/38 (63.2%) | 122/175 (69.7%) | 22/29 (75.9%) | 124/182 (68.1%) |
| Male | 14/38 (36.8%) | 53/175 (30.3%) | 7/29 (24.1%) | 58/182 (31.9%) |
| Smoking status | ||||
| Never | 9/38 (23.7%) | 27/175 (15.4%) | 7/29 (24.1%) | 26/182 (14.3%) |
| Former | 7/38 (18.4%) | 25/175 (14.3%) | 5/29 (17.2%) | 23/182 (12.6%) |
| Current | 1/38 (2.6%) | 6/175 (3.4%) | 2/29 (6.9%) | 5/182 (2.7%) |
| Not current (unknown if ever) | 21/38 (55.3%) | 117/175 (66.9%) | 15/29 (51.7%) | 128/182 (70.3%) |
| Education | ||||
| Elementary school | 3/38 (7.9%) | 2/175 (1.1%) | 2/29 (6.9%) | 4/182 (2.2%) |
| High school or equivalency | 11/38 (28.9%) | 47/175 (26.9%) | 7/29 (24.1%) | 51/182 (28.0%) |
| College | 19/38 (50.0%) | 87/175 (49.7%) | 10/29 (34.5%) | 78/182 (42.9%) |
| Post graduate | 4/38 (10.5%) | 32/175 (18.3%) | 9/29 (31.0%) | 45/182 (24.7%) |
| Other/Missing | 1/38 (2.6%) | 7/175 (4.0%) | 1/29 (3.4%) | 4/182 (2.2%) |
| Body Mass Index (weight.height−2) | 30.03±5.91 (N=38) | 30.86±6.32 (N=175) | 30.79±6.77 (N=29) | 29.57±5.63 (N=182) |
| # of prescription drugs | 6.03±3.77 (N=38) | 5.13±3.13 (N=175) | 6.41±3.38 (N=29) | 5.00±3.54 (N=182) |
| Taking Antidepressant/anti-anxiety meds | 16/38 (42.1%) | 33/175 (18.9%) | 16/29 (55.2%) | 36/182 (19.8%) |
| Folstein Mini Mental Status Exam | 26.37±2.82 (N=38) | 27.25±2.27 (N=175) | 26.52±2.35 (N=29) | 27.59±2.02 (N=182) |
| Self reported medical history/diagnoses | ||||
| Hypertension | 29/38 (76.3%) | 119/174 (68.4%) | 18/29 (62.1%) | 127/182 (69.8%) |
| Arthritis (past 6 months) | 8/38 (21.1%) | 42/175 (24.0%) | 8/29 (27.6%) | 35/181 (19.3%) |
| Fracture (hip or any other bone) | 7/38 (18.4%) | 57/175 (32.6%) | 9/29 (31.0%) | 55/182 (30.2%) |
| Heart attack/coronary/MI | 7/35 (20.0%) | 17/172 (9.9%) | 3/29 (10.3%) | 12/180 (6.7%) |
| Diabetes+ | 13/38 (34.2%) | 45/172 (26.2%) | 7/28 (25.0%) | 27/179 (15.1%) |
| Cancer (excluding skin) | 12/38 (31.6%) | 26/174 (14.9%) | 6/29 (20.7%) | 30/178 (16.9%) |
| Congestive heart failure | 3/37 (8.1%) | 8/170 (4.7%) | 3/29 (10.3%) | 10/178 (5.6%) |
| Stroke | 2/38 (5.3%) | 6/174 (3.4%) | 1/29 (3.4%) | 11/180 (6.1%) |
| Pacemaker | 0/37 (0.0%) | 5/162 (3.1%) | 0/28 (0.0%) | 3/172 (1.7%) |
Using a cutoff for Elevated Depressive Symptoms of CES-D >= 14
Prevalence of diabetes higher in high depressive symptoms in both PA and SA groups
Adherence rates for the center-based exercise sessions for participants in the PA groups for weeks 1–8, weeks 9–24 and 25-end were not statistically different for those with low depressive symptoms (% attendance: 71%, 62%, and 18% respectively) compared with those with high depressive symptoms (% attendance: 70%, 57%, and 15%). For the SA group, adherence to sessions did not statistically differ for the initial period from baseline to 6 months for those with low versus high depressive symptoms (% attendance: 71% versus 66%). Adherence for those with high depressive symptoms in the SA group (% attendance: 62%) was slightly lower (P = 0.038) than for those with low depressive symptoms (% attendance: 75%) in the 7 to 12 months period.
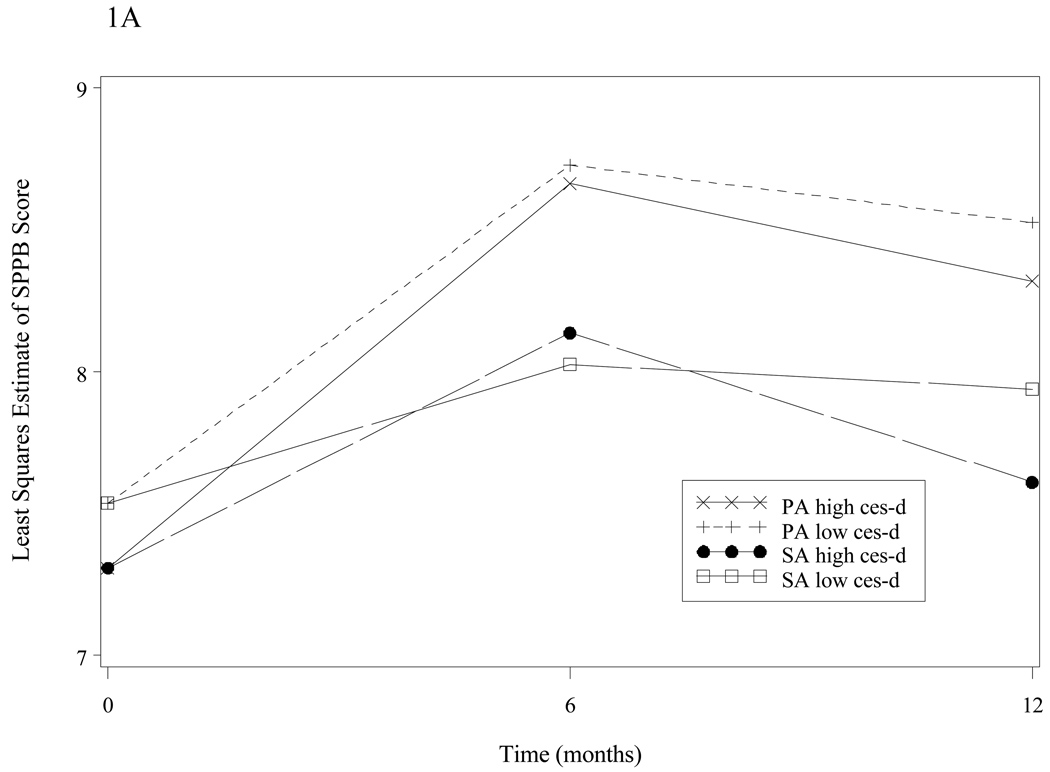
Figure 1A shows the adjusted mean SPPB score (expressed as the estimated least squares from repeated measures ANCOVA) according to depressive symptoms and intervention group membership. At baseline, participants with high depressive symptoms had lower SPPB scores compared with participants with low depressive symptoms in both the intervention and control groups. Among participants with low depressive scores, the PA group had significantly improved SPPB scores compared with the SA control group at the 6-month measure (adjusted difference +0.70; p < 0.001) and 12 month measure (adjusted difference +0.58; p = 0.004; p-value for average effect across all the follow-up visits is <0.001). In subjects with high depressive scores the adjusted difference in SPPB score between the two groups at 6 months was +0.76 (p=0.176) and at 12 months was +0.94 (p=0.116). Overall, there were no significant interactions between depressive symptoms and time (p=0.229) or depressive symptoms and intervention (p=0.689) for the SPPB outcome.
Figure 1.
Figure 1A: SPPB score over time, by intervention group and depressive symptoms group*
Figure 1A. The Short Physical Performance Battery (SPPB) score (adjusted differences) is shown over time, by intervention group and depressive symptoms group. Adjusted differences for low Centers for Epidemiological Studies Depression Scale (CES-D) groups were at 6 months −0.44, p=0.014, and 12 months −0.16, p=0.433; p-value for average effect across all the follow-up visits is 0.063. For high CES-D groups at 6 months: −0.07, p=0.864, and 12 months −0.12, p=0.813; p-value for average effect across all the follow-up visits is 0.820.
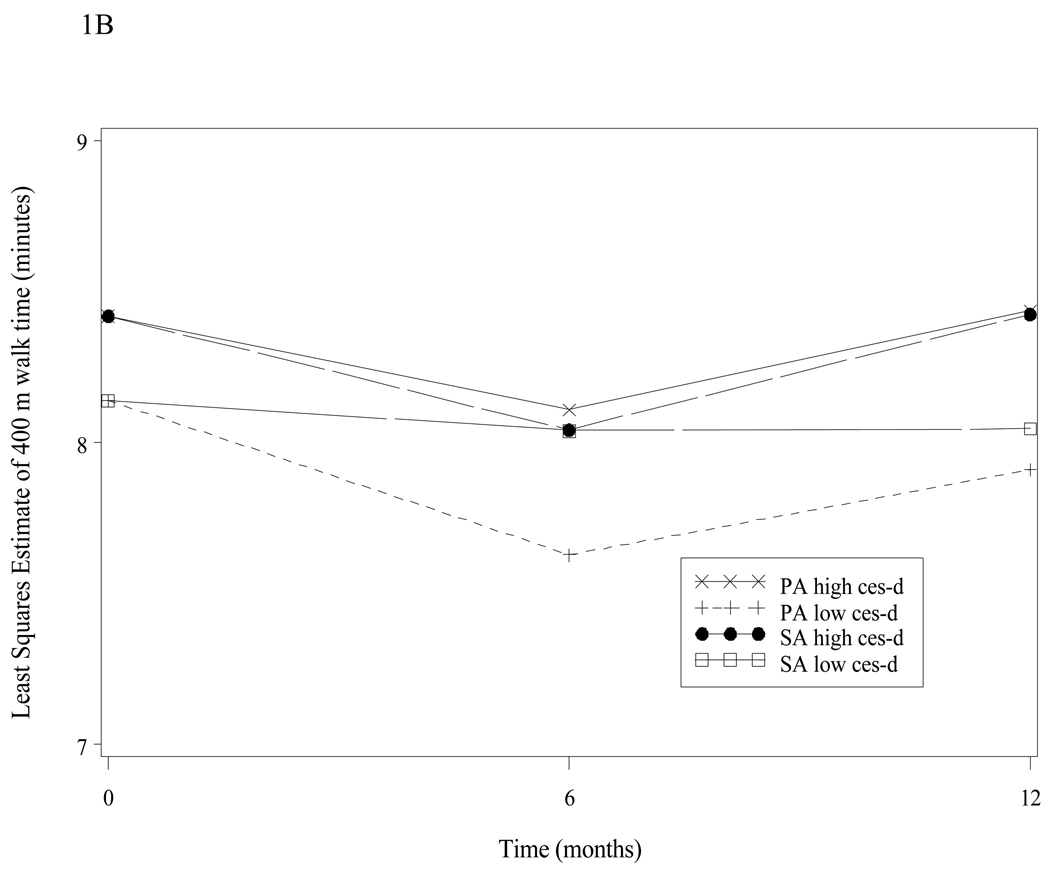
Figure 1B: 400 Meter Walk-Time over time by intervention group and depression group*
Figure 1B. The 400 meter walk (400 mw) time (adjusted differences) is shown over time, by intervention and depressive symptoms group. Adjusted differences for low CES-D groups were at 6 months −0.44, p=0.014, and 12 months −0.16, p=0.433; p-value for average effect across all the follow-up visits is 0.063. For high CES-D groups at 6 months: −0.07, p=0.864, and 12 months −0.12, p=0.813; p-value for average effect across all the follow-up visits is 0.820.
Time to complete the 400 mw (Figure 1B) showed an improvement trend over the 1 year period for those with low depressive symptom scores in the PA group versus SA control group (p = 0.089). Comparing the intervention group with the control group, the adjusted difference in 400 mw was significant at the 6 month interval (0.41 min.; p=0.021) but was not significant at the 1 year interval (0.14 min.; p=0.515) in those with low depressive symptoms. For those with high depressive symptoms there was no statistical improvement across all the follow-up visits despite improvements in raw score time differences (minutes) which demonstrated 3 to 5 second gains in the PA cohort (baseline: 8.380 +/−1.572, 6 months: 7.838 +/−1.785; 12 months: 8.023 +/−2.004) compared with the SA cohort (baseline: 8.481 +/−2.008; 6 months: 8.295 +/− 2.003; 12 months: 8.660 +/−2.331). Overall, there were no significant interactions between depressive symptoms and time (p=0.468) or depressive symptoms and intervention (p=0.388) for the 400 mw outcome.
There was no significant improvement in CES-D score over time as a result of participation in either intervention group (P=0.852). No significant changes in CES-D scores were found associated with either intervention when examined in participants with high depressive symptoms (p=0.385) and low depressive symptoms (p=0.670) over the 12 month study period.
DISCUSSION
The LIFE-P study is one of the first large multi-center randomized controlled trials to demonstrate improvements in physical performance measures after an exercise intervention in older sedentary adults (18). A post-hoc analysis from LIFE-P was performed to determine whether subjects with high depressive symptoms experience reduced benefits in physical performance measures after the exercise intervention. Results suggest that individuals with high depressive symptoms did not experience significantly diminished benefits in one physical performance measure, the SPPB, after the PA intervention. Although improvements for the high depressive symptoms groups did not reach statistical significance, raw clinical scores were well above the +0.5 point gain in SPPB score considered to represent clinically meaningful improvement in both the high and low depressive subgroups in the PA intervention arm (24).
Findings from this study are somewhat consistent with other exercise intervention studies that have demonstrated improvements in physical function in older adults with major depression (17) and in a general population of older adults with mixed depression scores (15). In the Fitness, Arthritis in Seniors Trial (FAST) trial, Pennix et al. demonstrated significant improvements in one performance measure, the 6 minute walking speed, for subjects with both low and high depressive symptoms in groups that underwent aerobic but not resistance training (15). In the present study, statistically significant improvements in a similar performance outcome (400 mw) occurred only for those in the low depressive symptom PA subgroup while improvements in another measure (SPPB) were similar between depressive subgroups. It is possible that such differences relate to the duration of the PA intervention which was shorter (12 versus 18 months) in the LIFE-P study versus the FAST trial as well as differences in PA programming between studies.
This analysis was underpowered to conclude whether or not the presence of high depressive symptoms negatively affects a measure of walking performance. However, it is possible that the 400 mw and the SPPB measures are differentially affected by the presence of depressive or other psychological symptoms. While the SPPB and 400 mw measures are considered to have moderate concordance (25), the latter is an endurance task that requires an individual to work harder and longer to achieve superior performance compared with the short performance tasks (26). One psychological factor that has been shown to differentially influence endurance versus short performance tasks in older adults and is strongly linked to depressive symptoms is self-efficacy (27, 28). A post-hoc analysis of self-efficacy and performance outcomes from LIFE-P demonstrated that poor self-efficacy, like depression, was related to both lower SPPB and 400 mw scores at baseline (29). It would be of future interest to compare both self-efficacy and depression as outcomes and determine how these variables interact and influence performance in both endurance and non-endurance tasks in the larger LIFE trial.
The hypothesis that depression scores (CES-D) would improve in those with high depressive symptoms as a result of the PA intervention was not supported in this exploratory study. This is in contrast to other studies that have demonstrated improvement in depression scores after exercise interventions in a general population of sedentary older adults (30) and in older adults with knee osteoarthritis (FAST trial) (15). In the FAST trial improvement in depression scores was seen in those with both high and low depressive symptoms at baseline after a program of aerobic exercise but not after a program of resistance exercise training (15). Among the differences between the FAST and LIFE-P studies that may explain differing depression outcomes include differences in study populations (older adults with knee osteoarthritis versus sedentary behavior), greater prevalence of depression in FAST vs. LIFE-P (22% vs. 16%), shorter study duration as well as lower dosage of aerobic intensity in LIFE-P which has been shown to influence depression outcomes (17).
This study has multiple limitations. Although a more liberal cutoff for CES-D was used to improve statistical power, the present study still lacked power due to relatively small numbers in the high depressive subgroups, thereby limiting conclusions regarding the relationship between depressive symptoms and physical performance outcomes. The ability to determine whether assignments utilizing a more liberal CES-D score were clinically relevant to mood impairment was limited by lack of a clinical assessment of depression in the LIFE-P study. Another important limitation of this study is that only participants that were able to complete the performance testing at each collection interval were included in the analysis. If characteristics of participants unable to complete the study differed from those included in the analysis this could bias the results and generalizability of the findings especially if those who were depressed were more likely to dropout. However, an analysis of missing data did not show significant differences between characteristics of participants who were unable to complete the study and those who were retained. Lastly, the potential for false positive results may occur when performing subgroup analyses in a clinical trial that is not designed to investigate the effects of an intervention based on depressive symptoms and findings must be confirmed in a larger intervention trial.
In conclusion, results of this post-hoc analysis from LIFE-P suggest that sedentary older adults with high depressive symptoms do not experience significantly diminished benefits in physical performance outcomes compared to subjects with low depressive symptoms after a physical activity intervention. Whether or not the presence of depressive symptoms exerts a small negative influence on endurance versus short functional performance tasks may warrant further exploration in the LIFE trial.
ACKNOWLEDGEMENTS
The Lifestyle Interventions and Independence for Elders (LIFE-P) Pilot Study is funded by a National Institutes on Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. Contributors to the LIFE-P study are acknowledged below in the Appendix: Research Investigators for Pilot Phase of LIFE.
Sponsor’s role: The funder of the original LIFE-P study had no direct role in this study.
Appendix
Research Investigators for Pilot Phase of LIFE
Cooper Institute, Dallas, TX:
Steven N. Blair, P.E.D. – Field Center Principal Investigator
Timothy Church, M.D., Ph.D., M.P.H. – Fielding Center Co-Principal Investigator
Jamile A. Ashmore, Ph.D.
Judy Dubreuil, M.S.
Georita Frierson, Ph.D.
Alexander N. Jordan, M.S.
Gina Morss, M.A.
Ruben Q. Rodarte, M.S.
Jason M. Wallace, M.P.H.
National Institute on Aging
Jack M. Guralnik, M.D., Ph.D. – Co-Principal Investigator of the Study
Evan C. Hadley, M.D.
Sergei Romashkan, M.D., Ph.D.
Stanford University, Palo Alto, CA
Abby C. King, Ph.D. – Field Center Principal Investigator
William L. Haskell, Ph.D. – Field Center Co-Principal Investigator
Leslie A. Pruitt, Ph.D.
Kari Abbott-Pilolla, M.S.
Karen Bolen, M.S.
Stephen Fortmann, M.D.
Ami Laws, M.D.
Carolyn Prosak, R.D.
Kristin Wallace, M.P.H.
Tufts University
Roger Fielding, Ph.D.
Miriam Nelson, Ph.D.
Dr. Fielding's contribution is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-4-401. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
University of California, Los Angeles, Los Angeles, CA
Robert M. Kaplan, Ph.D., M.A.
VA San Diego Healthcare System and University of California, San Diego, San Diego, CA
Erik J. Groessl, Ph.D.
University of Florida, Gainesville, FL
Marco Pahor, M.D. – Principal Investigator of the Study
Michael Perri, Ph.D.
Connie Caudle
Lauren Crump, M.P.H
Sarah Hayden
Latonia Holmes
Cinzia Maraldi, M.D.
Crystal Quirin
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, M.D., M.P.H. – Field Center Principal Investigator
Stephanie Studenski, M.D., M.P.H. – Field Center Co-Principal Investigator
Bret H. Goodpaster, Ph.D., M.S.
Nancy W. Glynn, Ph.D.
Erin K. Aiken, B.S.
Steve Anthony, M.S.
Sarah Beck (for recruitment papers only)
Judith Kadosh, B.S.N., R.N.
Piera Kost, B.A.
Mark Newman, M.S.
Jennifer Rush, M.P.H. (for recruitment papers only)
Roberta Spanos (for recruitment papers only)
Christopher A. Taylor, B.S.
Pam Vincent, C.M.A.
The Pittsburgh Field Center was partially supported by the Pittsburgh Claude D. Pepper Center P30 AG024827.
Wake Forest University, Winston-Salem, NC
Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator
Peter Brubaker, Ph.D.
Jamehl Demons, M.D.
Curt Furberg, M.D., Ph.D.
Jeffrey A. Katula, Ph.D., M.A.
Anthony Marsh, Ph.D.
Barbara J. Nicklas, Ph.D.
Jeff D. Williamson, M.D., M.P.H.
Rose Fries, L.P.M.
Kimberly Kennedy
Karin M. Murphy, B.S., M.T. (ASCP)
Shruti Nagaria, M.S.
Katie Wickley-Krupel, M.S.
Data Management, Analysis and Quality Control Center (DMAQC)
Michael E. Miller, Ph.D. – DMAQC Field Principal Investigator
Mark Espeland, Ph.D. – DMAQC Co-Principal Investigator
Fang-Chi Hsu, Ph.D.
Walter J. Rejeski, Ph.D.
Don P. Babcock, Jr., P.E.
Lorraine Costanza
Lea N. Harvin
Lisa Kaltenbach, M.S.
Wei Lang, Ph.D.
Wesley A. Roberson
Julia Rushing, M.S.
Scott Rushing
Michael P. Walkup, M.S.
The Wake Forest University Field Center is, in part, supported by the Claude D. Older American Independence Pepper Center #1 P30 AG21332.
Yale University
Thomas M. Gill, M.D.
Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging.
Footnotes
Conflicts of Interest: All authors have disclosed no evidence of financial or other conflicts of interest other than Dr. Blair who has disclosed receiving guest lecture honoraria, author related royalties from a previous textbook, and participates in grant activities that have been corporate and government funded.
Author Contributions: Drs. Matthews, Barry, and Patel were involved in study concept and design, data interpretation, manuscript preparation and critical revision. Dr. Hsu and Mike Walkup served a role in acquisition of data, statistical analysis, manuscript preparation and critical revision. Dr. Steven Blair’s role was PI of one of the clinical centers and participated in original study concept and design of the project, supervised conduct of the LIFE-P trial, and assisted in this manuscript preparation and critical revision.
REFERENCES
- 1.Stuck AE, Walthert JM, Nikolaus T, et al. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 2.Penninx BW, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 3.Lenze EJ, Schulz R, Martire LM, et al. The course of functional decline in older people with persistently elevated depressive symptoms: Longitudinal findings from the Cardiovascular Health Study. J Am Geriatr Soc. 2005;53:569–575. doi: 10.1111/j.1532-5415.2005.53202.x. [DOI] [PubMed] [Google Scholar]
- 4.Hassmen P, Koivula N, Uutela A. Physical exercise and psychological well-being: A population study in Finland. Prev Med. 2000;30:17–25. doi: 10.1006/pmed.1999.0597. [DOI] [PubMed] [Google Scholar]
- 5.Farmer ME, Locke BZ, Moscicki EK, et al. Physical activity and depressive symptoms: The NHANES I epidemiologic follow-up study. Am J Epidemiol. 1988;128:1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- 6.Pennix BW, Leveille S, Ferrucci L, et al. Exploring the effect of depression on physical disability: longitudinal evidence from the Established Populations for Epidemiologic Studies of the Elderly. Am J Public Health. 1999;89:1346–1353. doi: 10.2105/ajph.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everson-Rose S, Skarupski K, Bienias J, et al. Do depressive symptoms predict declines in physical performance in an elderly, biracial population? Psychosom Med. 2005;67:609–615. doi: 10.1097/01.psy.0000170334.77508.35. [DOI] [PubMed] [Google Scholar]
- 8.King AC, Rejeski J, Buchner DM. Physical activity interventions targeting older adults. A critical review and recommendations. Am J Prev Med. 1998;15:316–333. doi: 10.1016/s0749-3797(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 9.Manini TM, Pahor M. Physical activity and maintaining physical function in older adults. Br J Sports Med. 2009;43:28–31. doi: 10.1136/bjsm.2008.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw WS, Cronan TA, Christie MD. Predictors of attrition in health intervention research among older subjects with osteoarthritis. Health Psychol. 1994;13:421–431. doi: 10.1037//0278-6133.13.5.421. [DOI] [PubMed] [Google Scholar]
- 11.Brown WJ, Ford JH, Burton NW, et al. Prospective study of physical activity and depressive symptoms in middle-aged women. Am J Prev Med. 2005;29:265–272. doi: 10.1016/j.amepre.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Lindwall M, Rennemark M, Halling A, et al. Depression and exercise in elderly men and women: Findings from the Swedish National Study on Aging and Care. J Aging Phys Act. 2006;15:41–55. doi: 10.1123/japa.15.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 14.Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2001;56A:M497–M504. doi: 10.1093/gerona/56.8.m497. [DOI] [PubMed] [Google Scholar]
- 15.Pennix BW, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms: A comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol A Biol Sci Med Sci. 2002;57B(2):124–132. doi: 10.1093/geronb/57.2.p124. [DOI] [PubMed] [Google Scholar]
- 16.Brenes GA, Williamson JD, Messier SP, et al. Treatment of minor depression in older adults: A pilot study comparing sertraline and exercise. Aging Ment Health. 2007;11:61–68. doi: 10.1080/13607860600736372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh NA, Stavrinos TM, Scarbek Y, et al. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60A:768–776. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- 18.Pahor M, Blair SN, Epesland M, et al. The LIFE-P study investigators. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61A:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: Design and methods. Contemp Clin Trials. 2005;26:141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Stewart AL, Mills KM, King AC, et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J of Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 25.Sayers S, Guralnik J, Newman A, et al. Concordance and discordance between two measures of lower extremity function: 400 meter self-paced walk and SPPB. Aging Clin Exp Res. 2006;18:100–106. doi: 10.1007/BF03327424. [DOI] [PubMed] [Google Scholar]
- 26.Vasunilashorn S, Coppin A, Patel K, et al. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64(2):223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis-Berman J. Physical self-efficacy, perceived physical status, and depressive symptomatology in older adults. J Psychol. 1990;124:207. doi: 10.1080/00223980.1990.10543217. [DOI] [PubMed] [Google Scholar]
- 28.Maly M, Costigan P, Olney S. Self-efficacy mediates walking performance in older adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62(10):1142–1146. doi: 10.1093/gerona/62.10.1142. [DOI] [PubMed] [Google Scholar]
- 29.Rejeski WJ, King AC, Katula JA, et al. Physical activity in prefrail older adults: Confidence and satisfaction related to physical function. J Geronto B Psychol Sci Soc Sci. 2008;63(1):19–26. doi: 10.1093/geronb/63.1.p19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motl R, Konopack JF, McAuley E, et al. Depressive symptoms among older adults: Long-term reduction after a physical activity intervention. J Behav Med. 2005;28(4):385–394. doi: 10.1007/s10865-005-9005-5. [DOI] [PubMed] [Google Scholar]