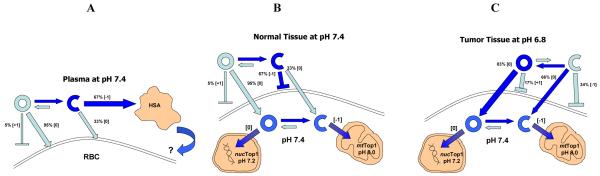
Chart 1. Predicted charge dynamics for BACPT lactone versus BACPT carboxylate.
Predominant species and dynamic pathways are denoted by dark blue. Hydrolysis of the pro-drug BACPTDP to BACPT is not shown and only the camptothecin E ring is represented. The predicted charged species for BACPT lactone (closed ring) are [+1] (basic), while those for BACPT carboxylate (open ring) are [−1] (acidic). Hence, the carboxylate is a weak acid, while the small amount of charged lactone is a weak base. Panel A: in plasma at pH 7.4, the equilibrium will favor conversion to the carboxylate form. The charged carboxylate species has a high affinity for human serum albumin, which further drives ring opening. Whether albumin-bound drug is terminally sequestered or still available (i.e., as in paclitaxel formulated in albumin nanoparticles; Abraxane ®), is not known. Counteracting the serum albumin pathway is the affinity of the uncharged BACPT lactone for red cell membranes where it is protected from ring opening. Panel B: in normal tissues, the equilibrium also favors conversion to the carboxylate form, which will yield a majority of charged species that cannot cross the plasma membrane. Panel C: in the acidic extracellular environment of solid tumors, equilibrium will favor the lactone form, most of which is not charged [0]. Furthermore, most of the carboxylate present will also be in an uncharged form that can enter cells. Once inside the tumor cell, the drug encounters physiological pH to again yield neutral and weak acid species. The neutral lactone will diffuse into the nucleus to inhibit nucTop1, while the acidic carboxylate will concentrate in basic mitochondrion to inhibit mtTop1. The kinetics of these charge interactions for BACPT remains a critical unknown and must be determined to assess the relative importance of each Top1 target to overall drug mechanism of action.