Abstract
Background
The American Cancer Society, the Centers for Disease Control and Prevention (CDC), the National Cancer Institute, and the North American Association of Central Cancer Registries (NAACCR) collaborate annually to provide updated information on cancer occurrence and trends in the United States. This year’s report highlights brain and other nervous system (ONS) tumors, including nonmalignant brain tumors, which became reportable on a national level in 2004.
Methods
Cancer incidence data were obtained from the National Cancer Institute, CDC, and NAACCR, and information on deaths was obtained from the CDC’s National Center for Health Statistics. The annual percentage changes in age-standardized incidence and death rates (2000 US population standard) for all cancers combined and for the top 15 cancers for men and for women were estimated by joinpoint analysis of long-term (1992–2007 for incidence; 1975–2007 for mortality) trends and short-term fixed interval (1998–2007) trends. Analyses of malignant neuroepithelial brain and ONS tumors were based on data from 1980–2007; data on nonmalignant tumors were available for 2004–2007. All statistical tests were two-sided.
Results
Overall cancer incidence rates decreased by approximately 1% per year; the decrease was statistically significant (P < .05) in women, but not in men, because of a recent increase in prostate cancer incidence. The death rates continued to decrease for both sexes. Childhood cancer incidence rates continued to increase, whereas death rates continued to decrease. Lung cancer death rates decreased in women for the first time during 2003–2007, more than a decade after decreasing in men. During 2004–2007, more than 213 500 primary brain and ONS tumors were diagnosed, and 35.8% were malignant. From 1987–2007, the incidence of neuroepithelial malignant brain and ONS tumors decreased by 0.4% per year in men and women combined.
Conclusions
The decrease in cancer incidence and mortality reflects progress in cancer prevention, early detection, and treatment. However, major challenges remain, including increasing incidence rates and continued low survival for some cancers. Malignant and nonmalignant brain tumors demonstrate differing patterns of occurrence by sex, age, and race, and exhibit considerable biologic diversity. Inclusion of nonmalignant brain tumors in cancer registries provides a fuller assessment of disease burden and medical resource needs associated with these unique tumors.
Since our first Report to the Nation, published in 1998, documented the first sustained decrease in cancer death rates since the 1930s (1), the American Cancer Society, the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) have collaborated annually to produce a report on the status of cancer in the United States. Each subsequent year, reports have updated information on trends in incidence and death rates and featured in-depth analyses of selected topics (2–12). The current report provides the latest information on trends for all cancers combined, childhood cancers, and for the top 15 cancers for each of the five major racial and ethnic groups by sex. Furthermore, this article presents a comprehensive assessment of the incidence of malignant and nonmalignant brain tumors in children and adults by race, sex, age group, and tumor histological type. National collection of nonmalignant brain tumors began in 2004 following the passage of Public Law 107-260, the Benign Brain Tumor Cancer Registries Amendment Act. The historical incidence, mortality, and survival by histological type, age, and era of diagnosis for malignant brain and other nervous system (ONS) tumors are presented.
Subjects and Methods
Cancers, Cancer Deaths, and Population Estimates
Population-based cancer registries that are NAACCR members and participate in the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program, and/or the CDC’s National Program of Cancer Registries were used to obtain information on newly diagnosed invasive cancers and benign and borderline brain tumors. Incident cases were classified by site and histology according to the International Classification of Diseases for Oncology (ICD-O) edition in use at the time of diagnosis, converted to the Third Edition coding (13) and categorized according to SEER site groups (14).
Incidence data were not available uniformly for every period, geographic area, and racial and ethnic group in the United States (Supplementary Table 1, available online). The longest continuous incidence data were available from the nine original SEER registries (SEER 9) covering about 10% of the US population. Long-term (1975–2007) trends based on data from the SEER 9 registries are included in Supplementary Table 2 (available online). Data providing better coverage of the US population (about 14%) were available from the SEER 13 registries and form the basis of our long-term incidence trend (1992–2007) analysis for all races and ethnicities combined (15). Beginning in 1995, following the advent of the National Program of Cancer Registries, coverage of the US population increased dramatically. Data from NAACCR covering 40 population-based cancer registries were used to assess short-term (1998–2007) trends. Data from 46 NAACCR population-based cancer registries were used to estimate 5-year (2003–2007) average annual age-standardized incidence rates for all races and ethnicities combined and for each of the five major racial and ethnic populations (white, black, Asian and Pacific Islander [API], American Indian/Alaska Native [AI/AN] who reside in counties covered by the Indian Health Service [IHS] Contract Health Service Delivery Area [CHSDA], and Hispanic). The 40 and 46 registries met NAACCR’s data quality criteria for every year included in the analysis; these registries covered 83.6% and 93% of the US population, respectively.
All primary brain and ONS tumors (ICD-O-3 codes C70.0–72.9 and C75.1–75.3, respectively), including malignant, borderline, and benign behaviors diagnosed in 2004–2007 were identified from 46 states in the NAACCR dataset. A neuropathologist reviewed the brain and ONS site and histology combinations and recommended excluding 1771 cases (0.8%) from analysis because of unlikely combinations. Consistent with the SEER site re-code convention, tumors coded to the nasal and nasopharyngeal regions also were excluded.
Data on approximately 76 000 malignant and 137 000 non-malignant brain and ONS tumors were analyzed. Within the brain and ONS, seven major histological groups were used in analyses (16,17). Tumors of neuroepithelial tissue were divided into eight specific histological subgroups (16). Tumors of neuroepithelial tissue coded as nonmalignant by registries, but for which only a malignant behavior code existed in ICD-O-3, were considered malignant. Consistent with previous practice, pilocytic astrocytomas were considered malignant. Malignant and papillary meningioma and meningeal sarcomatosis were categorized as malignant, whereas all other benign and uncertain or atypical meningioma histologies were categorized as nonmalignant, according to the ICD-O-3 behavior codes. Childhood brain and ONS tumors also were grouped using International Classification of Childhood Cancers (ICCC) definitions (18).
Cause of death is based on death certificate information reported to state vital statistics offices and compiled into a national file through the CDC National Center for Health Statistics National Vital Statistics System (19) and categorized according to SEER anatomic site groups (14) to maximize comparability among ICD and ICD-O versions. The underlying causes of death were selected according to the version of the ICD codes and selection rules in use at the time of death (ICD-6 to ICD-10) (20–24). We examined long-term (1975–2007) mortality trends for all races and ethnicities combined. Short-term (1998–2007) trends and 5-year (2003–2007) average annual age-standardized death rates were calculated for all cancer sites combined and for the top 15 cancer sites for men and women in each of the five major racial and ethnic populations. Death rates for the AI/AN population were based on deaths in counties served by IHS CHSDA because estimated rates based on CHSDA counties have been reported to be more accurate for this group (10,25).
County-level population estimates, summed to the state and national level, were used as denominators in calculations of incidence rates (26). The National Center for Health Statistics and the Census Bureau collaborate to provide NCI with bridged single-race annual population estimates, with annual reestimates calculated back to the most recent decennial census to accommodate multiracial data (27). The NCI makes slight modifications to the Hawaii population estimates based on additional local information (26).
For most states, population estimates as of July 1 of each year were used to calculate annual incidence and death rates. For Louisiana, Alabama, Mississippi, and Texas, where residents were displaced by Hurricanes Katrina and Rita, NCI made adjustments to the 2005 incidence data and underlying population data. The national total population estimates are not affected by these adjustments (further details are available at http://seer.cancer.gov/popdata/methods.html).
Statistical Analysis
Age-specific and age-standardized rates were expressed per 100 000 persons (or per 1 000 000 children), based on 2000 US standard population, and generated using SEER*Stat Software, Version 6.6.2 (http://www.seer.cancer.gov/seerstat) (28). Rates were suppressed if the numerator was less than 16 observations, consistent with our previous work (1–12).
Trends in age-standardized cancer incidence and US death rates were analyzed using joinpoint regression, which involves fitting a series of joined straight lines on a logarithmic scale to the trends in the annual age-standardized rates (http://www.srab.cancer.gov/joinpoint). We allowed a maximum of three joinpoints in models for the period 1992–2007 (Table 1), four joinpoints in models for the period 1975–2007 (Table 2 and Supplementary Table 2, available online), and up to two joinpoints for the period 1998–2007 for short-term fixed interval incidence (Table 3) and mortality analyses (Table 4). The joinpoint method is described in detail elsewhere (29). We present the long-term (1975–2007 and 1992–2007) trends in incidence using annual percent changes (APCs; ie, the slope of the line segment) based on observed data and APCs adjusted for reporting delays (which affect mostly recent years). Delay-adjustment is a statistical method to correct for unreported (delayed) or updated cancer cases. Delay-adjusted APCs, used in our description of results, are available only for long-term incidence data (Table 1 and Supplementary Table 2, available online) (30). The average APC (AAPC), a summary measure to compare fixed interval trends by race and ethnicity, is estimated as a geometric weighted average of the joinpoint APC trend analysis, with the weights equal to the lengths of each segment during the prespecified fixed interval (http://srab.cancer.gov/joinpoint/aapc.html) (31). The APC was suppressed if the numerator was less than 10 cancers for any year, consistent with our previous methods (1–12).
Table 1.
Surveillance, Epidemiology, and End Results (SEER) cancer incidence rate trends with joinpoint analyses (up to three joinpoints allowed) for 1992–2007 for the top 15 cancers, by sex, for all races*
| Sex/cancer site or type | Joinpoint analyses (1992–2007)† |
|||||||||
| Trend 1 |
Trend 2 |
Trend 3 |
Trend 4 |
AAPC‡ |
||||||
| Years | APC§ | Years | APC§ | Years | APC§ | Years | APC§ | 1998–2007 | 2003–2007 | |
| All sites‖ | ||||||||||
| Both sexes | 1992–1994 | −3.2¶ | 1994–1999 | 0.4 | 1999–2007 | −1.0¶ | −0.8# | −1.0# | ||
| Delay adjusted | 1992–1994 | −3.1¶ | 1994–1999 | 0.4 | 1999–2007 | −0.8¶ | −0.7# | −0.8# | ||
| Men | 1992–1995 | −4.5¶ | 1995–2000 | 0.2 | 2000–2007 | −1.4¶ | −1.1# | −1.4# | ||
| Delay adjusted | 1992–1995 | −4.4¶ | 1995–2001 | 0.1 | 2001–2005 | −1.9¶ | 2005–2007 | 0.3 | −0.7# | −0.8 |
| Women | 1992–1998 | 0.8¶ | 1998–2007 | −0.8¶ | −0.8# | −0.8# | ||||
| Delay adjusted | 1992–1998 | 0.8¶ | 1998–2007 | −0.6¶ | −0.6# | −0.6# | ||||
| Children (age 0–14 y) | 1992–2007 | 0.4 | 0.4 | 0.4 | ||||||
| Delay adjusted | 1992–2007 | 0.5 | 0.5# | 0.5# | ||||||
| Children (age 0–19 y) | 1992–2007 | 0.5¶ | 0.5# | 0.5# | ||||||
| Delay adjusted | 1992–2007 | 0.6¶ | 0.6# | 0.6# | ||||||
| Top 15 cancers for men** | ||||||||||
| Prostate | 1992–1995 | −11¶ | 1995–2001 | 1.8¶ | 2001–2005 | −4.3¶ | 2005–2007 | 2.2 | −0.9 | −1.1 |
| Delay adjusted | 1992–1995 | −11¶ | 1995–2001 | 1.8¶ | 2001–2005 | −4.2¶ | 2005–2007 | 3.0 | −0.6 | −0.6 |
| Lung and bronchus | 1992–2007 | −2.1¶ | −2.1# | −2.1# | ||||||
| Delay adjusted | 1992–2007 | −2¶ | −2.0# | −2.0# | ||||||
| Colon and rectum | 1992–1995 | −2.7¶ | 1995–1998 | 1.7 | 1998–2007 | −3.0¶ | −3.0# | −3.0# | ||
| Delay adjusted | 1992–1995 | −2.6¶ | 1995–1998 | 1.7 | 1998–2007 | −2.9¶ | −2.9# | −2.9# | ||
| Urinary bladder | 1992–2007 | −0.2 | −0.2 | −0.2 | ||||||
| Delay adjusted | 1992–2007 | −0.1 | −0.1 | −0.1 | ||||||
| Non-Hodgkin lymphoma | 1992–2007 | −0.1 | −0.1 | −0.1 | ||||||
| Delay adjusted | 1992–2007 | 0.0 | 0.0 | 0.0 | ||||||
| Melanoma of the skin | 1992–2007 | 2.2¶ | 2.2# | 2.2# | ||||||
| Delay adjusted | 1992–2007 | 2.4¶ | 2.4# | 2.4# | ||||||
| Kidney and renal pelvis | 1992–2007 | 1.9¶ | 1.9# | 1.9# | ||||||
| Delay adjusted | 1992–2007 | 2.0¶ | 2.0# | 2.0# | ||||||
| Oral cavity and pharynx | 1992–2007 | −1.5¶ | −1.5# | −1.5# | ||||||
| Delay adjusted | 1992–2007 | −1.4¶ | −1.4# | −1.4# | ||||||
| Leukemia | 1992–2007 | −0.6¶ | −0.6# | −0.6# | ||||||
| Delay adjusted | 1992–2007 | 0.1 | 0.1 | 0.1 | ||||||
| Pancreas | 1992–2007 | 0.2 | 0.2# | 0.2# | ||||||
| Delay adjusted | 1992–2003 | 0.0 | 2003–2007 | 1.9¶ | 0.8# | 1.9# | ||||
| Stomach | 1992–2007 | −1.9¶ | −1.9# | −1.9# | ||||||
| Delay adjusted | 1992–2007 | −1.9¶ | −1.9# | −1.9# | ||||||
| Liver and intrahepatic bile duct | 1992–2007 | 3.2¶ | 3.2# | 3.2# | ||||||
| Delay adjusted | 1992–2007 | 3.4¶ | 3.4# | 3.4# | ||||||
| Esophagus | 1992–2007 | −0.1 | −0.1 | −0.1 | ||||||
| Delay adjusted | 1992–2007 | 0.0 | 0.0 | 0.0 | ||||||
| Brain and other nervous system | 1992–2007 | −0.6¶ | −0.6# | −0.6# | ||||||
| Delay adjusted | 1992–2007 | −0.4¶ | −0.4# | −0.4# | ||||||
| Myeloma | 1992–2007 | −0.2 | −0.2 | −0.2 | ||||||
| Delay adjusted | 1992–2007 | 0.2 | 0.2 | 0.2 | ||||||
| Top 15 cancers for women** | ||||||||||
| Breast | 1992–1999 | 1.1¶ | 1999–2007 | −1.8¶ | −1.5# | −1.8# | ||||
| Delay adjusted | 1992–1999 | 1.1¶ | 1999–2007 | −1.8¶ | −1.4# | −1.8# | ||||
| Lung and bronchus | 1992–1998 | 0.6 | 1998–2007 | −0.6¶ | −0.6# | −0.6# | ||||
| Delay adjusted | 1992–1997 | 0.7 | 1997–2007 | −0.3¶ | −0.3# | −0.3# | ||||
| Colon and rectum | 1992–1995 | −1.9¶ | 1995–1998 | 2.0 | 1998–2007 | −2.3¶ | −2.3# | −2.3# | ||
| Delay adjusted | 1992–1995 | −1.8¶ | 1995–1998 | 2.0 | 1998–2007 | −2.2¶ | −2.2# | −2.2# | ||
| Corpus and uterus, NOS | 1992–2007 | −0.3¶ | −0.3# | −0.3# | ||||||
| Delay adjusted | 1992–2007 | −0.2¶ | −0.2# | −0.2# | ||||||
| Non-Hodgkin lymphoma | 1992–2004 | 1.2¶ | 2004–2007 | −1.8 | 0.2 | −1.1 | ||||
| Delay adjusted | 1992–2004 | 1.3¶ | 2004–2007 | −1.2 | 0.4 | −0.6 | ||||
| Thyroid | 1992–1998 | 3.8¶ | 1998–2007 | 6.4¶ | 6.4# | 6.4# | ||||
| Delay adjusted | 1992–1998 | 3.8¶ | 1998–2007 | 6.6¶ | 6.6# | 6.6# | ||||
| Melanoma of the skin | 1992–1997 | 3.9¶ | 1997–2007 | 1.5¶ | 1.5# | 1.5# | ||||
| Delay adjusted | 1992–2007 | 2.2¶ | 2.2# | 2.2# | ||||||
| Ovary‖ | 1992–2001 | −0.6¶ | 2001–2007 | −2.0¶ | −1.5# | −2.0# | ||||
| Delay adjusted‖ | 1992–1996 | −1.5 | 1996–2001 | 0.2 | 2001–2004 | −2.9 | 2004–2007 | −0.4 | −1.0 | −1.0 |
| Kidney and renal pelvis | 1992–2007 | 2.3¶ | 2.3# | 2.3# | ||||||
| Delay adjusted | 1992–1998 | 1.2 | 1998–2007 | 3.0¶ | 3.0# | 3.0# | ||||
| Pancreas | 1992–2007 | 0.4¶ | 0.4# | 0.4# | ||||||
| Delay adjusted | 1992–2000 | −0.1 | 2000–2007 | 1.3¶ | 1.0# | 1.3# | ||||
| Leukemia | 1992–2007 | −0.3 | −0.3 | −0.3 | ||||||
| Delay adjusted | 1992–2007 | 0.5¶ | 0.5# | 0.5# | ||||||
| Urinary bladder | 1992–2004 | −0.2 | 2004–2007 | −2.7¶ | −1.0# | −2.1# | ||||
| Delay adjusted | 1992–2004 | −0.2 | 2004–2007 | −2.2 | −0.9# | −1.7# | ||||
| Cervix uteri | 1992–2007 | −2.9¶ | −2.9# | −2.9# | ||||||
| Delay adjusted | 1992–2007 | −2.8¶ | −2.8# | −2.8# | ||||||
| Oral cavity and pharynx | 1992–2007 | −1.2¶ | −1.2# | −1.2# | ||||||
| Delay adjusted | 1992–2007 | −1.1¶ | −1.1# | −1.1# | ||||||
| Brain and other nervous system | 1992–2007 | −0.2 | −0.2 | −0.2 | ||||||
| Delay adjusted | 1992–2007 | 0.0 | 0.0 | 0.0 | ||||||
AAPC = average annual percent change; APC = annual percent change; NOS = not otherwise specified. Source: SEER-13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, New Mexico, the Alaska Native Tumor Registry, rural Georgia, and the metropolitan areas of San Francisco, Los Angeles, San Jose-Monterey, Detroit, Atlanta, and Seattle-Puget Sound). Nonadjusted rates and rates that were adjusted for delays in reporting are shown.
Joinpoint analyses with up to three joinpoints yielding up to four trend segments (Trend 1–Trend 4) were based on rates per 100 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, >85 years, Census P25-1130). Joinpoint analysis used the Joinpoint Regression Program, Version 3.4.3. April 2010, Surveillance Research Program, National Cancer Institute.
AAPC is a weighted average of the APCs calculated by joinpoint.
APC is based on rates that were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, >85 years, Census P25–1130).
All sites exclude myelodysplastic syndromes and borderline tumors; ovary excludes borderline tumors.
APC is statistically significantly different from zero (two-sided t test, P < .05).
AAPC is statistically significantly different from zero (two-sided Z test, P < .05).
The top 15 cancers were selected based on the sex-specific age-standardized incidence rates for 2003–2007 for all races combined and listed in rank order.
Table 2.
US cancer death rate trends with joinpoint analyses (up to four joinpoints allowed) for 1975–2007 for the top 15 cancers, by sex, for all races*
| Sex/cancer site or type | Joinpoint analyses (1975–2007)† |
|||||||||||
| Trend 1 |
Trend 2 |
Trend 3 |
Trend 4 |
Trend 5 |
AAPC‡ |
|||||||
| Years | APC§ | Years | APC§ | Years | APC§ | Years | APC§ | Years | APC§ | 1998–2007 | 2003–2007 | |
| All sites | ||||||||||||
| Both sexes | 1975–1990 | 0.5‖ | 1990–1993 | −0.3 | 1993–2001 | −1.1‖ | 2001–2007 | −1.6‖ | −1.4¶ | −1.6¶ | ||
| Men | 1975–1979 | 1.0‖ | 1979–1990 | 0.3‖ | 1990–1993 | −0.4 | 1993–2001 | −1.5‖ | 2001–2007 | −1.9‖ | −1.8¶ | −1.9¶ |
| Women | 1975–1990 | 0.6‖ | 1990–1994 | −0.1 | 1994–2002 | −0.8‖ | 2002–2007 | −1.5‖ | −1.2¶ | −1.5¶ | ||
| Children ages 0–14 | 1975–1997 | −2.9‖ | 1997–2007 | −1.0‖ | −1.0¶ | −1.0¶ | ||||||
| Children ages 0–19 | 1975–1996 | −2.7‖ | 1996–2007 | −1.2‖ | −1.2¶ | −1.2¶ | ||||||
| Top 15 cancers for men# | ||||||||||||
| Lung and bronchus | 1975–1978 | 2.4‖ | 1978–1984 | 1.2‖ | 1984–1991 | 0.3‖ | 1991–2005 | −1.9‖ | 2005–2007 | −3.0‖ | −2.1¶ | −2.5¶ |
| Prostate | 1975–1987 | 0.9‖ | 1987–1991 | 3.0‖ | 1991–1994 | −0.5 | 1994–2005 | −4.1‖ | 2005–2007 | −2.6‖ | −3.7¶ | −3.3¶ |
| Colon and rectum | 1975–1984 | −0.1 | 1984–1990 | −1.4‖ | 1990–2002 | −2.0‖ | 2002–2005 | −4.3‖ | 2005–2007 | −2.1 | −2.8¶ | −3.2¶ |
| Pancreas | 1975–1986 | −0.8‖ | 1986–2002 | −0.3‖ | 2002–2007 | 0.7‖ | 0.3 | 0.7¶ | ||||
| Leukemia | 1975–1996 | −0.2‖ | 1996–2007 | −0.9‖ | −0.9¶ | −0.9¶ | ||||||
| Non-Hodgkin lymphoma | 1975–1991 | 2.7‖ | 1991–1997 | 1.6‖ | 1997–2007 | −3.0‖ | −3.0¶ | −3.0¶ | ||||
| Esophagus | 1975–1985 | 0.7‖ | 1985–1994 | 1.2‖ | 1994–2005 | 0.5‖ | 2005–2007 | −1.2 | 0.1 | −0.4 | ||
| Liver and intrahepatic bile duct | 1975–1979 | 0.3 | 1979–1987 | 2.3‖ | 1987–1996 | 3.9‖ | 1996–1999 | 0.6 | 1999–2007 | 2.3‖ | 2.1¶ | 2.3¶ |
| Urinary bladder | 1975–1983 | −1.4‖ | 1983–1987 | −2.7‖ | 1987–1993 | 0.1 | 1993–2003 | −0.6‖ | 2003–2007 | 0.5 | −0.1 | 0.5 |
| Kidney and renal pelvis | 1975–1991 | 1.1‖ | 1991–2002 | −0.1 | 2002–2007 | −1.3‖ | −0.8¶ | −1.3¶ | ||||
| Stomach | 1975–1987 | −2.3‖ | 1987–1991 | −0.9 | 1991–2007 | −3.5‖ | −3.5¶ | −3.5¶ | ||||
| Brain and other nervous system | 1975–1977 | 4.4 | 1977–1982 | −0.4 | 1982–1991 | 1.3‖ | 1991–2007 | −1.0‖ | −1.0¶ | −1.0¶ | ||
| Myeloma | 1975–1994 | 1.5‖ | 1994–2007 | −1.1‖ | −1.1¶ | −1.1¶ | ||||||
| Melanoma of the skin | 1975–1989 | 2.3‖ | 1989–2007 | 0.2‖ | 0.2¶ | 0.2¶ | ||||||
| Oral cavity and pharynx | 1975–1977 | 0.7 | 1977–1993 | −2.0‖ | 1993–2000 | −2.9‖ | 2000–2007 | −1.2‖ | −1.6¶ | −1.2¶ | ||
| Top 15 cancers for women# | ||||||||||||
| Lung and bronchus | 1975–1982 | 6.0‖ | 1982–1990 | 4.2‖ | 1990–1995 | 1.7‖ | 1995–2003 | 0.3‖ | 2003–2007 | −0.9‖ | −0.2 | −0.9¶ |
| Breast | 1975–1990 | 0.4‖ | 1990–2007 | −2.2‖ | −2.2¶ | −2.2¶ | ||||||
| Colon and rectum | 1975–1984 | −1.0‖ | 1984–2001 | −1.8‖ | 2001–2007 | −3.2‖ | −2.7¶ | −3.2¶ | ||||
| Pancreas | 1975–1984 | 0.8‖ | 1984–2007 | 0.1‖ | 0.1¶ | 0.1¶ | ||||||
| Ovary | 1975–1982 | −1.2‖ | 1982–1992 | 0.3‖ | 1992–1998 | −1.2‖ | 1998–2002 | 0.8 | 2002–2007 | −1.7‖ | −0.6 | −1.7¶ |
| Non-Hodgkin lymphoma | 1975–1995 | 2.2‖ | 1995–1998 | −0.5 | 1998–2007 | −3.6‖ | −3.6¶ | −3.6¶ | ||||
| Leukemia | 1975–1980 | 0.7 | 1980–2000 | −0.4‖ | 2000–2007 | −1.6‖ | −1.3¶ | −1.6¶ | ||||
| Corpus and uterus, NOS | 1975–1989 | −1.6‖ | 1989–1997 | −0.7‖ | 1997–2007 | 0.3‖ | 0.3¶ | 0.3¶ | ||||
| Brain and other nervous system | 1975–1992 | 1.0‖ | 1992–2007 | −1.1‖ | −1.1¶ | −1.1¶ | ||||||
| Liver and intrahepatic bile duct | 1975–1978 | −1.5 | 1978–1988 | 1.4‖ | 1988–1995 | 3.9‖ | 1995–2000 | 0.3 | 2000–2007 | 1.6‖ | 1.3¶ | 1.6¶ |
| Myeloma | 1975–1993 | 1.5‖ | 1993–2001 | −0.4 | 2001–2007 | −2.3‖ | −1.7¶ | −2.3¶ | ||||
| Stomach | 1975–1987 | −2.8‖ | 1987–1990 | −0.3 | 1990–2007 | −2.7‖ | −2.7¶ | −2.7¶ | ||||
| Kidney and renal pelvis | 1975–1992 | 1.3‖ | 1992–2007 | −0.6‖ | −0.6¶ | −0.6¶ | ||||||
| Cervix uteri | 1975–1982 | −4.4‖ | 1982–1996 | −1.6‖ | 1996–2003 | −3.8‖ | 2003–2007 | −0.5 | −2.4¶ | −0.5 | ||
| Urinary bladder | 1975–1986 | −1.7‖ | 1986–2007 | −0.4‖ | −0.4¶ | −0.4¶ | ||||||
AAPC = average annual percent change; APC = annual percent change; NOS = not otherwise specified. Source: National Center for Health Statistics public-use data file for the total US, 1975–2007.
Joinpoint analyses with up to four joinpoints yielding up to five trend segments (Trend 1–Trend 5) are based on rates per 100 000 persons and were age adjusted to the 2000 US standard population (19 age groups—Census P25–1130). Joinpoint Regression Program, Version 3.4.3. April 2010, Surveillance Research Program, National Cancer Institute.
AAPC is the average annual percent change and is a weighted average of the APCs calculated by Joinpoint.
APC is based on rates that were age-adjusted to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, >85 years, Census P25–1130).
APC is statistically significantly different from zero (two-sided t test, P < .05).
AAPC is statistically significantly different from zero (two-sided Z test, P < .05).
The top 15 cancers were selected based on the sex-specific age-standardized death rates for 2003–2007 for all races combined and listed in rank order.
Table 3.
Incidence rates for 2003–2007 and short-term fixed-interval trends for 1998–2007 for the top 15 cancers by sex, race, and ethnicity, for areas in the United States with high-quality incidence data*
| Sex/cancer site or type† | All races/ethnicities |
White‡ |
Black‡ |
API‡ |
AI/AN (CHSDA)‡ |
Hispanic‡ |
Non-Hispanic‡ |
|||||||||||||||
| Rank | Rate§ | 1998–2007 AAPC‖ | 2003–2007 AAPC‖ | Rank | Rate§ | 1998–2007 AAPC‖ | Rank | Rate§ | 1998–2007 AAPC‖ | Rank | Rate§ | 1998–2007 AAPC‖ | Rank | Rate§ | 1998–2007 AAPC‖ | Rank | Rate§ | 1998–2007 AAPC‖ | Rank | Rate§ | 1998–2007 AAPC‖ | |
| All sites¶ | ||||||||||||||||||||||
| Both sexes | 471.4 | −0.6# | −0.6# | 470.6 | −0.7# | 484.3 | −0.8# | 298.7 | −0.9# | 385.5 | −0.7# | 368.2 | −1.0# | 480.7 | −0.5# | |||||||
| Men | 552.5 | −0.8# | −1.3# | 544.9 | −0.9# | 623.1 | −1.4# | 332.3 | −1.4# | 424.6 | −1.3# | 426.1 | −1.4# | 563.5 | −0.6 | |||||||
| Women | 414.7 | −0.5# | −0.5# | 418.8 | −0.5# | 392.9 | −0.5 | 278.1 | −0.3# | 359.2 | −0.2 | 331.2 | −0.6# | 422.3 | −0.4# | |||||||
| Children age 0−14 y | 15.5 | 0.6# | 0.6# | 16.0 | 0.3 | 12.2 | 1.4# | 12.7 | 0.8 | 12.1 | −0.4 | 15.6 | 0.6 | 15.5 | 0.6# | |||||||
| Children age 0−19 y | 17.1 | 0.7# | 0.7# | 17.8 | 0.5# | 13.0 | 1.2# | 13.6 | 1.3 | 13.5 | 0.6 | 17.0 | 1.0# | 17.2 | 0.7# | |||||||
| Men | ||||||||||||||||||||||
| Prostate | 1 | 153.5 | −0.4 | −0.2 | 1 | 143.8 | −0.7 | 1 | 230.0 | −1.0 | 1 | 81.0 | −1.5 | 1 | 101.5 | −2.3# | 1 | 128.0 | −2.0# | 1 | 155.7 | −0.3 |
| Lung and bronchus | 2 | 84.9 | −2.0# | −2.6# | 2 | 84.3 | −2.0# | 2 | 103.5 | −2.5# | 2 | 49.9 | −2.2# | 2 | 70.2 | −1.2# | 3 | 48.0 | −2.9# | 2 | 87.8 | −1.9# |
| Colon and rectum | 3 | 57.1 | −3.0# | −4.0# | 3 | 56.1 | −3.2# | 3 | 67.2 | −1.8# | 3 | 42.8 | −2.3# | 3 | 51.9 | −2.3# | 2 | 49.2 | −1.9# | 3 | 57.8 | −3.0# |
| Urinary bladder | 4 | 37.7 | −1.0# | −1.7# | 4 | 39.7 | −1.0# | 5 | 18.8 | −0.2 | 6 | 15.3 | −0.6 | 5 | 17.5 | −0.9 | 4 | 20.9 | −1.4# | 4 | 39.0 | −0.9# |
| Non-Hodgkin lymphoma | 5 | 23.2 | −0.1 | −0.8 | 6 | 23.7 | 0.0 | 6 | 16.8 | −0.3 | 7 | 14.5 | −1.4# | 6 | 16.3 | −0.4 | 5 | 19.5 | −0.7 | 6 | 23.5 | 0.0 |
| Melanoma of the skin | 6 | 23.1 | 2.6# | 2.6# | 5 | 25.4 | 2.5# | 26 | 1.1 | −0.9 | 20 | 1.6 | 0.3 | 13 | 6.5 | 0.0 | 16 | 4.6 | −0.5 | 5 | 24.9 | 2.8# |
| Kidney and renal pelvis | 7 | 20.1 | 2.6# | 2.6# | 7 | 20.2 | 2.6# | 4 | 21.6 | 3.0# | 9 | 9.6 | 2.7# | 4 | 26.9 | 2.5# | 6 | 18.9 | 1.8# | 7 | 20.3 | 2.7# |
| Oral cavity and pharynx | 8 | 16.0 | −0.3# | −0.3# | 9 | 16.0 | 0.0 | 7 | 16.5 | −2.9# | 8 | 10.4 | −1.7# | 9 | 13.2 | −2.9 | 11 | 10.5 | −2.2# | 8 | 16.6 | −0.1 |
| Leukemia | 9 | 16.0 | −0.7 | −1.9# | 8 | 16.3 | −0.7 | 12 | 12.1 | −1.3# | 11 | 8.6 | −1.9# | 10 | 11.8 | −1.0 | 9 | 11.8 | −1.3# | 9 | 16.2 | −0.6 |
| Pancreas | 10 | 13.2 | 0.6# | 0.6# | 10 | 13.0 | 0.7# | 8 | 16.5 | −0.2 | 10 | 9.6 | 0.0 | 11 | 10.9 | 1.3 | 10 | 11.4 | −0.1 | 10 | 13.4 | 0.7# |
| Stomach | 11 | 9.7 | −2.2# | −2.2# | 12 | 8.7 | −2.4# | 9 | 16.4 | −2.2# | 5 | 17.2 | −2.8# | 7 | 14.5 | −2.1 | 8 | 14.1 | −3.3# | 11 | 9.3 | −2.3# |
| Liver and intrahepatic bile duct | 12 | 9.3 | 3.5# | 3.5# | 14 | 8.2 | 3.4# | 10 | 13.5 | 4.8# | 4 | 21.6 | −0.3 | 8 | 14.3 | 2.5 | 7 | 16.4 | 2.4# | 13 | 8.8 | 3.4# |
| Esophagus | 13 | 8.7 | 0.2 | 0.2 | 11 | 8.7 | 0.8# | 14 | 10.0 | −5.0# | 14 | 4.0 | −1.7 | 14 | 6.4 | −4.2 | 15 | 5.6 | −1.4# | 12 | 9.0 | 0.3 |
| Brain and other nervous system | 14 | 7.9 | −0.4# | −0.4# | 13 | 8.4 | −0.3 | 15 | 4.7 | −0.2 | 13 | 4.0 | −1.5 | 16 | 4.9 | −1.4 | 13 | 6.1 | −0.8# | 14 | 8.1 | −0.3# |
| Myeloma | 15 | 7.0 | −0.1 | −1.5 | 16 | 6.5 | −0.2 | 11 | 13.3 | 0.1 | 15 | 3.9 | 0.2 | 12 | 6.8 | −4.8# | 12 | 6.6 | −0.4 | 16 | 7.0 | −0.1 |
| Larynx | 16 | 7.0 | −2.7# | −2.7# | 15 | 6.8 | −2.5# | 13 | 11.0 | −2.7# | 18 | 2.3 | −6.2# | 15 | 5.4 | −3.8# | 14 | 5.9 | −3.7# | 15 | 7.1 | −2.5# |
| Thyroid | 18 | 5.1 | 6.0# | 6.0# | 18 | 5.4 | 6.1# | 19 | 2.7 | 5.6# | 12 | 4.6 | 4.6# | 18 | 3.2 | −6.1 | 18 | 4.0 | 4.9# | 18 | 5.3 | 6.3# |
| Women | ||||||||||||||||||||||
| Breast | 1 | 120.7 | −1.3# | −0.7 | 1 | 121.9 | −1.4# | 1 | 114.6 | −0.4 | 1 | 82.3 | −0.1 | 1 | 88.2 | −1.0# | 1 | 91.0 | −0.9# | 1 | 123.4 | −1.2# |
| Lung and bronchus | 2 | 55.6 | 0.0 | −0.6 | 2 | 57.0 | 0.1 | 2 | 51.8 | −0.4 | 3 | 27.7 | 0.3 | 2 | 50.6 | 1.4 | 3 | 27.1 | −0.4 | 2 | 57.9 | 0.2 |
| Colon and rectum | 3 | 42.4 | −2.3# | −2.9# | 3 | 41.4 | −2.5# | 3 | 50.7 | −1.7# | 2 | 32.5 | −1.7# | 3 | 42.2 | −1.5 | 2 | 34.9 | −1.9# | 3 | 43.0 | −2.3# |
| Corpus and uterus, NOS | 4 | 23.9 | 0.0 | 0.0 | 4 | 24.4 | −0.1 | 4 | 21.3 | 1.6# | 4 | 15.8 | 1.6# | 4 | 20.0 | 1.2 | 4 | 19.4 | 0.6# | 4 | 24.3 | 0.0 |
| Non-Hodgkin lymphoma | 5 | 16.3 | −0.2 | −1.0# | 6 | 16.8 | −0.3 | 6 | 11.6 | 0.3 | 6 | 10.3 | −1.4# | 6 | 14.3 | 1.0 | 5 | 15.1 | −0.1 | 5 | 16.4 | −0.2 |
| Thyroid | 6 | 15.2 | 7.2# | 7.2# | 7 | 15.8 | 7.5# | 11 | 9.3 | 6.9# | 5 | 15.5 | 5.9# | 8 | 10.3 | 4.4# | 6 | 14.9 | 6.6# | 7 | 15.3 | 7.6# |
| Melanoma of the skin | 7 | 15.0 | 3.2# | 3.2# | 5 | 16.9 | 3.2# | 28 | 1.0 | 1.1 | 21 | 1.3 | −1.3 | 15 | 5.0 | 6.9# | 17 | 4.4 | 0.9 | 6 | 16.3 | 3.5# |
| Ovary¶ | 8 | 12.9 | −1.7# | −2.3# | 8 | 13.3 | −1.8# | 9 | 9.7 | −1.2 | 8 | 9.2 | −1.1# | 7 | 11.3 | −2.7 | 8 | 11.4 | −1.1# | 8 | 13.0 | −1.8# |
| Kidney and renal pelvis | 9 | 10.5 | 3.1# | 3.1# | 9 | 10.6 | 3.2# | 7 | 11.0 | 3.3# | 14 | 4.7 | 3.1# | 5 | 16.5 | 2.3# | 9 | 11.0 | 2.9# | 9 | 10.5 | 3.1# |
| Pancreas | 10 | 10.2 | 0.7# | 0.7# | 11 | 10.0 | 0.8# | 5 | 13.6 | 0.1 | 10 | 7.8 | −0.8 | 9 | 10.0 | −0.5 | 10 | 9.8 | −0.1 | 10 | 10.3 | 0.7# |
| Leukemia | 11 | 9.7 | −0.3 | −1.3# | 12 | 9.9 | −0.4 | 13 | 7.7 | −0.8 | 12 | 5.7 | −1.1 | 11 | 7.6 | −1.0 | 12 | 8.3 | −1.0# | 12 | 9.7 | −0.5 |
| Urinary bladder | 12 | 9.6 | −1.0# | −2.0# | 10 | 10.0 | −1.1# | 14 | 6.7 | −0.8 | 15 | 3.9 | −1.5 | 18 | 4.5 | 2.0 | 14 | 5.5 | −1.7# | 11 | 9.9 | −0.9# |
| Cervix uteri | 13 | 8.1 | −2.7# | −1.3# | 13 | 7.7 | −2.5# | 8 | 10.7 | −4.3# | 11 | 7.4 | −3.8# | 10 | 9.7 | −2.5 | 7 | 12.5 | −3.8# | 13 | 7.6 | −2.8# |
| Oral cavity and pharynx | 14 | 6.1 | −0.6# | −0.6# | 15 | 6.1 | −0.5# | 15 | 5.5 | −2.1# | 13 | 5.1 | −1.6 | 14 | 5.4 | 0.7 | 18 | 4.0 | 0.1 | 14 | 6.3 | −0.5# |
| Brain and other nervous system | 15 | 5.8 | −0.5# | −0.6# | 14 | 6.1 | −0.3# | 17 | 3.6 | −0.5 | 16 | 3.1 | 0.4 | 19 | 4.0 | −0.1 | 16 | 4.8 | −1.0# | 15 | 5.9 | −0.5# |
| Stomach | 16 | 4.8 | −1.3# | −1.3# | 16 | 4.1 | −1.5# | 12 | 8.4 | −1.8# | 7 | 9.7 | −3.1# | 12 | 7.3 | −2.1 | 11 | 8.6 | −2.0# | 17 | 4.4 | −1.4# |
| Myeloma | 17 | 4.6 | −1.0# | −2.1# | 17 | 4.0 | −1.1# | 10 | 9.6 | −0.8 | 17 | 2.7 | −1.5 | 16 | 5.0 | −4.5 | 15 | 4.8 | −1.8 | 16 | 4.6 | −0.9# |
| Liver and intrahepatic bile duct | 18 | 3.2 | 1.8# | 1.8# | 18 | 2.8 | 1.5# | 16 | 3.9 | 2.5# | 9 | 8.1 | 0.1 | 13 | 7.2 | 4.8# | 13 | 6.2 | 0.9# | 18 | 2.9 | 1.6# |
AAPC = average annual percent change; APC = annual percent change; AI/AN = American Indian/Alaska Native; API = Asian/Pacific Islander; CHSDA = Contract Health Services Delivery Area; IHS = Indian Health Service; NOS = not otherwise specified. Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program areas reported by North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time periods. 2003–2007 rates for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (46 states): Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming. 2003–2007 AAPCs and 1998–2007 AAPCs for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (40 states): Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Massachusetts, Metropolitan Atlanta, Michigan, Minnesota, Missouri, Montana, Nebraska, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, Wyoming.
Cancers are sorted in descending order according to sex-specific rates for all races/ethnicities. More than 15 cancers may appear under men and women to include the top 15 cancers in every race/ethnicity group.
White, black, API, and AI/AN (CHSDA counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Incidence rates are per 100 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130).
AAPC is the average annual percent change and is a weighted average of the annual percent change (APC calculated by Joinpoint over the time period 1998–2007 unless otherwise noted. Joinpoint analyses with up to two joinpoints are based on rates per 100 000 persons and were age standardized to the 2000 US standard population (19 age groups: under 1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130). Joinpoint Regression Program, Version 3.4.3. April 2010, Surveillance Research Program National Cancer Institute.
For all sites, myelodysplastic syndromes are included for the rate calculations but not for the APC calculations; they are excluded from cancer-specific analysis. Ovary excludes borderline tumors.
AAPC is statistically significantly different from zero (two-sided Z test, P < .05).
Table 4.
Death rates for 2003–2007 and fixed-interval trends for 1998–2007 for the top 15 cancers* by sex, race, and ethnicity in the United States†
| Sex/cancer site or type* | All races/ethnicities |
White‡ |
Black‡ |
API‡ |
AI/AN (CHSDA counties)‡ |
Hispanic‡,§ |
Non-Hispanic‡,§ |
|||||||||||||||
| Rank | Rate‖ | 1998–2007 AAPC¶ | 2003–2007 AAPC¶ | Rank | Rate‖ | 1998–2007 AAPC¶ | Rank | Rate‖ | 1998–2007 AAPC¶ | Rank | Rate‖ | 1998–2007 AAPC¶ | Rank | Rate‖ | 1998–2007 AAPC¶ | Rank | Rate‖ | 1998–2007 AAPC¶ | Rank | Rate‖ | 1998–2007 AAPC¶ | |
| All malignant cancers | ||||||||||||||||||||||
| Both sexes | 183.8 | −1.3# | −1.6# | 182.4 | −1.2# | 224.2 | −2.0# | 110.8 | −1.6# | 156.7 | −0.5 | 122.1 | −1.8# | 188.3 | −1.2# | |||||||
| Men | 225.4 | −1.8# | −1.8# | 222.5 | −1.7# | 296.5 | −2.6# | 134.2 | −2.0# | 183.7 | −1.0# | 150.6 | −2.5# | 230.8 | −1.7# | |||||||
| Women | 155.4 | −1.1# | −1.4# | 155.0 | −1.0# | 180.6 | −1.4# | 94.1 | −1.2# | 138.0 | −0.2 | 102.3 | −1.3# | 159.3 | −1.0# | |||||||
| Children age 0–14 y | 2.4 | −1.0# | −3.0# | 2.4 | −0.9# | 2.3 | −0.7 | 2.1 | −2.0 | 1.5 | ** | 2.5 | −1.3 | 2.4 | −1.0# | |||||||
| Children ages 0–19 y | 2.6 | −1.3# | −2.9# | 2.7 | −1.1# | 2.5 | −1.5# | 2.3 | −2.0# | 2.1 | 1.9 | 2.8 | −0.7 | 2.6 | −1.5# | |||||||
| Men | ||||||||||||||||||||||
| Lung and bronchus | 1 | 68.8 | −2.1# | −2.1# | 1 | 68.3 | −1.9# | 1 | 87.5 | −2.9# | 1 | 36.7 | −1.7# | 1 | 48.1 | −2.2# | 1 | 32.5 | −3.3# | 1 | 71.7 | −1.9# |
| Prostate | 2 | 24.7 | −3.9# | −3.9# | 2 | 22.8 | −3.8# | 2 | 54.2 | −4.2# | 4 | 10.6 | −3.1# | 2 | 20.0 | −1.6 | 2 | 18.8 | −3.8# | 2 | 25.0 | −3.8# |
| Colon and rectum | 3 | 21.2 | −2.8# | −3.3# | 3 | 20.6 | −2.9# | 3 | 30.5 | −1.9# | 3 | 13.2 | −3.1# | 3 | 19.2 | −1.8 | 3 | 15.6 | −2.6# | 3 | 21.6 | −2.7# |
| Pancreas | 4 | 12.3 | 0.2 | 0.9# | 4 | 12.2 | 0.4# | 4 | 15.4 | −0.7# | 6 | 8.2 | 0.0 | 5 | 9.9 | 3.5 | 5 | 9.1 | −0.4 | 4 | 12.6 | 0.4# |
| Leukemia | 5 | 9.7 | −0.8# | −1.2# | 5 | 10.0 | −0.6# | 8 | 8.4 | −1.3# | 8 | 4.9 | −1.0 | 9 | 5.8 | 0.1 | 8 | 6.0 | −1.7# | 5 | 9.9 | −0.7# |
| Non-Hodgkin lymphoma | 6 | 8.7 | −3.0# | −3.0# | 6 | 9.1 | −3.0# | 11 | 6.0 | −2.7# | 7 | 5.5 | −2.8# | 10 | 5.3 | −3.7 | 7 | 6.4 | −3.4# | 6 | 8.9 | −2.9# |
| Esophagus | 7 | 7.8 | 0.2 | 0.2 | 8 | 7.9 | 0.9# | 7 | 8.9 | −4.4# | 9 | 3.2 | −2.0 | 8 | 6.4 | 0.0 | 10 | 4.0 | −2.1# | 7 | 8.0 | 0.3# |
| Liver and intrahepatic bile duct | 8 | 7.7 | 2.2# | 2.2# | 9 | 7.0 | 2.2# | 5 | 11.1 | 2.6# | 2 | 14.7 | −1.1# | 4 | 10.9 | 2.2 | 4 | 11.3 | 1.0# | 9 | 7.4 | 2.2# |
| Urinary bladder | 9 | 7.5 | −0.1 | −0.1 | 7 | 7.9 | 0.0 | 13 | 5.4 | −0.3 | 12 | 2.6 | −2.8# | 13 | 3.0 | †† | 11 | 3.9 | −0.9 | 8 | 7.8 | 0.0 |
| Kidney and renal pelvis | 10 | 5.9 | −0.7# | −0.7# | 10 | 6.0 | −0.7# | 12 | 6.0 | −0.6# | 11 | 2.6 | 0.2 | 7 | 8.8 | −0.7 | 9 | 5.2 | −0.3 | 10 | 5.9 | −0.7# |
| Stomach | 11 | 5.3 | −3.5# | −3.5# | 12 | 4.6 | −3.7# | 6 | 10.7 | −3.5# | 5 | 9.4 | −3.6# | 6 | 9.2 | −1.7 | 6 | 8.0 | −3.7# | 12 | 5.1 | −3.7# |
| Brain and other nervous system | 12 | 5.2 | −1.3# | −1.3# | 11 | 5.6 | −1.1# | 15 | 3.1 | −1.5# | 13 | 2.3 | −1.2 | 14 | 2.7 | 3.1 | 13 | 3.2 | −1.3# | 11 | 5.4 | −1.1# |
| Myeloma | 13 | 4.4 | −1.0# | −1.0# | 14 | 4.2 | −0.9# | 9 | 8.1 | −1.7# | 14 | 2.0 | −0.9 | 11 | 4.2 | −0.6 | 12 | 3.3 | −1.4 | 13 | 4.5 | −0.9# |
| Melanoma of the skin | 14 | 4.0 | 0.3 | 0.3 | 13 | 4.5 | 0.5 | 21 | 0.5 | 1.2 | 20 | 0.4 | †† | 16 | 1.6 | †† | 16 | 1.0 | −1.5 | 14 | 4.3 | 0.6# |
| Oral cavity and pharynx | 15 | 3.9 | −1.5# | −1.5# | 15 | 3.7 | −1.1# | 10 | 6.3 | −3.1# | 10 | 3.1 | −2.8# | 12 | 3.5 | −3.2 | 14 | 2.5 | −3.8# | 15 | 4.0 | −1.3# |
| Larynx | 16 | 2.2 | −2.4# | −2.4# | 16 | 2.0 | −2.2# | 14 | 4.6 | −2.8# | 16 | 0.8 | −1.8 | 15 | 1.9 | †† | 15 | 1.8 | −6.4# | 16 | 2.3 | −2.2# |
| Soft tissue including heart | 17 | 1.4 | −0.9# | 1.2# | 18 | 1.5 | −0.7 | 16 | 1.4 | −2.9# | 15 | 1.0 | −0.6 | 18 | 1.0 | †† | 17 | 1.0 | −2.7# | 17 | 1.5 | −0.7 |
| Women | ||||||||||||||||||||||
| Lung and bronchus | 1 | 40.6 | −0.2 | −0.2 | 1 | 41.6 | −0.1 | 1 | 39.6 | −0.3 | 1 | 18.5 | −0.6 | 1 | 33.3 | 1.2 | 2 | 14.4 | −0.4 | 1 | 42.6 | 0.0 |
| Breast | 2 | 24.0 | −2.0# | −2.0# | 2 | 23.4 | −2.0# | 2 | 32.4 | −1.4# | 2 | 12.2 | −1.0# | 2 | 17.6 | 1.1 | 1 | 15.3 | −1.9# | 2 | 24.7 | −1.8# |
| Colon and rectum | 3 | 14.9 | −2.6# | −2.8# | 3 | 14.4 | −2.7# | 3 | 21.0 | −2.7# | 3 | 9.9 | −1.5# | 3 | 12.9 | −2.3 | 3 | 10.5 | −1.5# | 3 | 15.2 | −2.6# |
| Pancreas | 4 | 9.4 | 0.3# | 0.3# | 4 | 9.1 | 0.4# | 4 | 12.4 | −0.2 | 4 | 6.9 | 0.1 | 4 | 8.0 | 1.6 | 4 | 7.5 | 0.2 | 4 | 9.5 | 0.3# |
| Ovary | 5 | 8.6 | −0.5# | −1.7# | 5 | 8.9 | −0.5# | 6 | 7.2 | −1.1# | 7 | 4.9 | 0.6 | 5 | 6.8 | 0.2 | 5 | 6.0 | −0.1 | 5 | 8.8 | −0.4# |
| Non-Hodgkin lymphoma | 6 | 5.5 | −3.4# | −3.1# | 6 | 5.7 | −3.4# | 11 | 3.9 | −2.6# | 8 | 3.5 | −3.4# | 7 | 4.6 | −0.7 | 8 | 4.4 | −2.8# | 6 | 5.6 | −3.5# |
| Leukemia | 7 | 5.4 | −1.4# | −1.4# | 7 | 5.6 | −1.3# | 9 | 5.0 | −1.5# | 9 | 2.9 | −2.1# | 9 | 3.9 | †† | 9 | 3.9 | −1.7# | 7 | 5.5 | −1.1# |
| Corpus and uterus, NOS | 8 | 4.1 | 0.2# | 0.2# | 8 | 3.9 | 0.1 | 5 | 7.2 | 0.8# | 10 | 2.5 | 1.5 | 13 | 2.9 | †† | 11 | 3.0 | −0.9 | 8 | 4.2 | 0.3# |
| Brain and other nervous system | 9 | 3.5 | −1.2# | −1.2# | 9 | 3.8 | −1.1# | 16 | 2.0 | −1.6# | 12 | 1.6 | 1.2 | 17 | 1.6 | †† | 13 | 2.4 | −0.7 | 9 | 3.6 | −1.1# |
| Liver and intrahepatic bile duct | 10 | 3.2 | 1.4# | 1.4# | 10 | 3.0 | 1.6# | 12 | 3.9 | 0.4 | 5 | 6.4 | −1.2 | 6 | 6.6 | 1.4 | 6 | 5.2 | 0.5 | 10 | 3.1 | 1.3# |
| Myeloma | 11 | 2.9 | −1.5# | −2.2# | 12 | 2.7 | −1.4# | 7 | 5.8 | −2.3# | 13 | 1.4 | −1.8 | 12 | 3.0 | −4.2 | 12 | 2.5 | −1.0 | 11 | 2.9 | −1.6# |
| Stomach | 12 | 2.7 | −3.0# | −3.0# | 13 | 2.4 | −3.1# | 8 | 5.0 | −4.0# | 6 | 5.6 | −3.5# | 8 | 4.2 | −5.9# | 7 | 4.6 | −3.0# | 13 | 2.6 | −3.2# |
| Kidney and renal pelvis | 13 | 2.7 | −0.6# | −0.6# | 11 | 2.7 | −0.5# | 14 | 2.7 | −0.2 | 15 | 1.2 | −0.1 | 10 | 3.8 | −2.5 | 14 | 2.4 | −0.5 | 12 | 2.7 | −0.5# |
| Cervix uteri | 14 | 2.4 | −2.2# | −0.7 | 15 | 2.2 | −2.0# | 10 | 4.4 | −3.5# | 11 | 2.1 | −4.6# | 11 | 3.4 | −2.4 | 10 | 3.1 | −2.3# | 14 | 2.4 | −2.3# |
| Urinary bladder | 15 | 2.2 | −0.6# | −0.6# | 14 | 2.2 | −0.4# | 13 | 2.7 | −1.1# | 16 | 0.9 | −0.4 | 19 | 0.9 | †† | 15 | 1.3 | −0.7 | 15 | 2.3 | −0.4 |
| Esophagus | 17 | 1.7 | −1.4# | −1.4# | 17 | 1.6 | −0.7# | 15 | 2.5 | −4.6# | 17 | 0.8 | −0.7 | 15 | 1.7 | †† | 18 | 0.8 | −3.5# | 17 | 1.7 | −1.2# |
| Oral cavity and pharynx | 18 | 1.4 | −2.1# | −2.1# | 18 | 1.4 | −1.9# | 17 | 1.5 | −3.8# | 14 | 1.2 | −0.9 | 16 | 1.6 | †† | 19 | 0.8 | −2.0 | 18 | 1.5 | −2.0# |
| Gallbladder | 20 | 0.8 | −2.1# | −2.1# | 20 | 0.8 | −2.3# | 19 | 0.9 | −0.6 | 18 | 0.8 | −7.3# | 14 | 2.4 | †† | 16 | 1.2 | −5.3# | 20 | 0.7 | −1.9# |
Cancers are sorted in descending order according to sex-specific rates for all races/ethnicities. More than 15 cancers may appear under men and women to include the top 15 cancers in every race/ethnicity group.
AAPC = average annual percent change; AI/AN = American Indian/Alaska Native; APC = annual percent change; API = Asian/Pacific Islander; CHSDA = Contract Health Services Delivery Area; IHS = Indian Health Service; NOS = not otherwise specified. Source: National Center for Health Statistics mortality file for the total United States.
White, black, API, and AI/AN (CHSDA counties) populations include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Data for Hispanic and non-Hispanic exclude the District of Columbia, Maine, Minnesota, New Hampshire, and North Dakota.
Incidence rates are per 100 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130).
AAPC is a weighted average of the APCs calculated by Joinpoint over the time period 1998–2007 unless otherwise noted. Joinpoint analyses with up to two joinpoints are based on rates per 100 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130). Joinpoint Regression Program, Version 3.4.3. April 2010, Surveillance Research Program, National Cancer Institute.
AAPC is statistically significantly different from zero (two-sided Z test, P < .05).
Joinpoint cannot process records with weight variable less than or equal to zero.
Statistic could not be calculated. The AAPC is based on fewer than 10 cancer cases for at least 1 year within the time interval.
In describing long- and short-term trends with estimates of APC and AAPC, the terms “increase” or “decrease” signify that the slope (APC or AAPC) of the trend was statistically significant (P < .05) using a t test (APC) or Z test (AAPC). For non-statistically significant trends, we used terms such as “level,” “stable,” “non-statistically significant increase,” or “non-statistically significant decrease.” All statistical tests were two-sided.
Results
Long-term Incidence Trends for All Races Combined, 1992–2007
Trend analysis showed that overall cancer incidence rates for all racial and ethnic groups combined decreased by 0.8% per year during the most recent period, 2003–2007 (Table 1); a statistically significant decrease of 0.6% per year was noted in women, whereas a non-statistically significant decrease of 0.8% per year was noted in men that was influenced by a recent (2005–2007) non-statistically significant increase in prostate cancer incidence. When prostate cancer was excluded from the trend analysis, there was a statistically significant decrease in cancer incidence for all sites combined (data not shown). Incidence for prostate and breast cancers, two of the most frequently diagnosed cancers, showed possible changing trends. Cancer of the prostate showed a non-statistically significant annual increase of 3.0% in 2005–2007, after a statistically significant decrease in 2001–2005. The trend analysis of breast cancer in women showed a decrease from 1999 until 2007. However, inspection of the annual breast cancer incidence rates during this period (data not shown) revealed that, after a sharp decrease in rates in 2002–2003, the lower rates subsequently remained stable. The cancer rates among children (0–19 years of age) showed an increase of 0.6% per year for both the most recent 5-year period (2003–2007) and the entire period (1992–2007).
During the period 2003–2007, incidence rates for five of the 15 most common cancers among men demonstrated a statistically significant decrease: lung and bronchus (lung), colon and rectum (colorectal), oral cavity and pharynx (oral), stomach, and malignant brain tumors. Trends in four cancers among men (melanoma of the skin, kidney and renal pelvis [kidney], pancreas, and liver and intrahepatic bile duct [liver]) showed statistically significant increases during the period 2003–2007, whereas trends for prostate, urinary bladder (bladder), and esophageal cancers and leukemia, myeloma, and non-Hodgkin lymphoma did not demonstrate a statistically significant increase or decrease. Among women, statistically significant increasing trends were noted in three of the four cancers that were increasing in men (kidney, pancreas, melanoma of the skin); leukemia and thyroid cancer also increased. Statistically significant decreasing trends for women included cancers of the breast, lung, colon and rectum, corpus uteri (uterus), cervix uteri (cervix), bladder, and oral cavity. No statistically significant trends in non-Hodgkin lymphoma, malignant brain tumors, and cancer of the ovary were observed.
Long-term Mortality Trends for All Races Combined, 1975–2007
Since the early 1990s, overall cancer death rates have shown a statistically significant decreasing trend among both men and women; whereas for children, cancer death rates have decreased since the mid-1970s (Table 2). Trends in death rates during the most recent 10- and 5-year periods (1998–2007 and 2003–2007) continued to decrease for seven of the top 15 cancer types in both men and women (colon and rectum, brain, stomach, and kidney cancers, and non-Hodgkin lymphoma, leukemia, and myeloma); for cancers of the lung, prostate, and oral cavity in men; and for breast and bladder cancers in women. In contrast, during the corresponding time intervals, death rates from liver cancer and melanoma of the skin in men and those for liver and pancreatic cancers in women continued to exhibit statistically significant increases. Notably, lung cancer death rates in women revealed a statistically significant decrease during the period 2003–2007, following long-term increases during the period 1975–2003, and cervical cancer death rates stabilized after decreasing for many decades. It also is noteworthy that long-term trends in death rates may mask important changes during the shorter term. For example, the 10-year AAPC (1998–2007) for lung cancer in women showed a small non-statistically significant decrease of 0.2% that was composed of a statistically significant increase of 0.3% from 1995 to 2003, followed by a statistically significant decrease of 0.9% from 2003 to 2007 (Table 2).
Cancer Incidence Rates, 2003–2007, and Short-term Fixed Interval Trends by Race and Ethnicity, 1998–2007
Black men had the highest cancer incidence rate for 2003–2007 of any racial and ethnic group (Table 3). Except among Hispanics, the top three cancer sites for men in each population group were, in rank order, prostate, lung, and colorectal cancers; among Hispanics, the colorectal cancer rate was slightly higher than the rate of lung cancer. Among women, white women had the highest overall incidence rates. Breast cancer was the most commonly diagnosed cancer among women regardless of race and ethnicity. Lung and colorectal cancers ranked second and third (respectively) among women of all races combined and for white, black, and AI/AN women. However, these rankings were reversed among API and Hispanic women. For all populations, cancer of the uterus ranked fourth. Beyond the top three cancer sites for men and top four for women, cancer rankings varied by race and ethnicity.
Incidence rates for all cancer sites combined decreased between 1998 and 2007 in both men and women in all populations; although the decrease was non-statistically significant among black or AI/AN women (Table 3). Childhood (ages 0–19 years) cancer incidence increased in all populations, although the increase was non-statistically significant for API and AI/AN children. Prostate cancer incidence showed a statistically significant decrease among AI/AN and Hispanic men. Breast cancer incidence rates decreased in all women, but the decrease was of smaller magnitude and non-statistically significant for black and API women. Among men, lung cancer incidence rates decreased for all populations; among women, no statistically significant change was observed in any racial or ethnic group. Colorectal cancer rates decreased among both men and women in all population groups, but the decrease was non-statistically significant for AI/AN women. Cancer of the uterus increased among black, API, and Hispanic women but not among white women. Incidence rates of esophageal cancer increased among white men but decreased among blacks and Hispanics.
Cancer Death Rates, 2003–2007, and Short-term Trends by Race and Ethnicity, 1998–2007
Overall cancer death rates from 1998–2007 decreased for all race, ethnic, and sex groups except AI/AN women, among whom the decrease was non-statistically significant (Table 4). However, the largest average annual percentage decrease occurred in black and Hispanic men, approximately 2.5% per year. During the corresponding time interval, overall cancer death rates also showed a statistically significant decrease of 1%–2% per year for children aged 0–19 years in each racial and ethnic group, except in Hispanics and AI/AN, in whom rates were stable. Similarly, death rates in each racial and ethnic group decreased for each of the three major cancers in men and women (lung, colorectal, prostate, or breast), except the trends for prostate and colon cancers among AI/AN men and for lung cancer among women of all racial groups were non-statistically significant. A statistically significant increase in liver cancer death rates was noted among white men and women and black and Hispanic men, whereas the increase in pancreatic cancer death rates was noted only in white men and women.
Brain and ONS Tumors
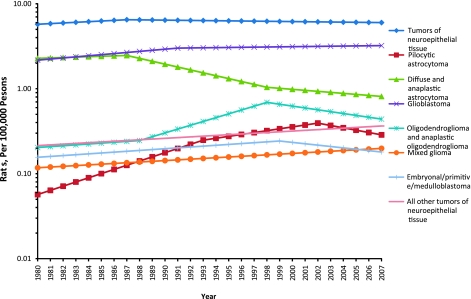
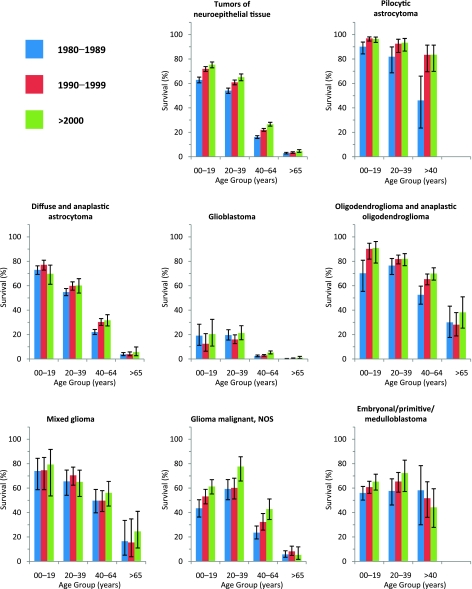
The distribution of malignant, benign, and borderline brain and ONS tumors during the period 2004–2007 is shown for adults in Table 5 and for children in Table 6. Nonmalignant tumors were about twice as common as malignant tumors among adults (aged ≥20 years). Women had an overall brain tumor incidence rate of 26.55 per 100 000 persons; men had a corresponding rate of 22.37. Tumors of neuroepithelial tissue were the most common histological group of malignant brain tumor, occurring more frequently in men than women. Glioblastoma, which occurred 1.6 times more frequently in men, was the most common subtype of neuroepithelial tumor. The most common type of nonmalignant adult brain tumor was meningioma, which was 2.3 times more common in women than in men, with an incidence rate of 12.42 per 100 000 vs 5.46 per 100 000 persons, respectively. The rate of meningioma in women was by far the highest incidence rate for any type of brain tumor in either sex. In contrast to neuroepithelial tumors, 94.9% of which were malignant, only 2.1% of meningiomas were malignant.
Table 5.
Age-standardized incidence rates and counts of adult (age ≥20 years) brain and other nervous system tumors including lymphomas by major histological groupings, sex, and behavior (nonmalignant, malignant), North American Association of Central Cancer Registries (NAACCR) combined, 2004–2007*
| Histological group† | Malignant, benign and borderline malignancy |
Malignant | Benign and borderline malignancy‡ | Percent malignant | ||||||
| Men |
Women |
Men and women |
||||||||
| Rate§ | Count | Rate§ | Count | Rate§ | Count | Median age | Count | Count | ||
| Brain and other nervous system | 22.37 | 83 281 | 26.55 | 115 508 | 24.55 | 198 789 | 60.0 | 66 968 | 131 821 | 33.7 |
| Tumors of neuroepithelial tissue | 9.38 | 35 275 | 6.47 | 27 813 | 7.81 | 63 088 | 59.0 | 59 888 | 3200 | 94.9 |
| Pilocytic astrocytoma | 0.14 | 550 | 0.13 | 534 | 0.14 | 1084 | 34.0 | 1084 | 0 | 100.0 |
| Diffuse and anaplastic astrocytoma‖ | 1.44 | 5433 | 1.04 | 4334 | 1.22 | 9767 | 53.0 | 9767 | 0 | 100.0 |
| Glioblastoma | 5.54 | 20 592 | 3.51 | 15 597 | 4.43 | 36 189 | 64.0 | 36 189 | 0 | 100.0 |
| Oligodendroglioma and anaplastic oligodendroglioma | 0.60 | 2338 | 0.47 | 1870 | 0.53 | 4208 | 45.0 | 4208 | 0 | 100.0 |
| Mixed glioma | 0.32 | 1227 | 0.22 | 866 | 0.27 | 2093 | 42.0 | 2093 | 0 | 100.0 |
| Glioma malignant, NOS | 0.44 | 1629 | 0.34 | 1478 | 0.39 | 3107 | 61.0 | 3107 | 0 | 100.0 |
| Embryonal/primitive/medulloblastoma | 0.09 | 353 | 0.07 | 274 | 0.08 | 627 | 33.0 | 625 | †† | 99.7 |
| All other tumors of neuroepithelial tissue¶ | 0.81 | 3153 | 0.70 | 2860 | 0.75 | 6013 | 45.0 | 2815 | 3198 | 46.8 |
| Tumors of cranial and spinal nerves | 2.25 | 8749 | 2.24 | 9498 | 2.24 | 18 247 | 54.0 | 190 | 18 057 | 1.0 |
| Nerve sheath | 2.25 | 8747 | 2.24 | 9495 | 2.24 | 18 242 | 54.0 | 190 | 18 052 | 1.0 |
| Acoustic neuromas | 1.45 | 5618 | 1.46 | 6240 | 1.45 | 11 858 | 55.0 | 32 | 11 826 | 0.3 |
| All other nerve sheath | 0.80 | 3129 | 0.77 | 3255 | 0.79 | 6384 | 53.0 | 158 | 6226 | 2.5 |
| Tumors of meninges | 5.79 | 20 907 | 12.67 | 56 339 | 9.50 | 77 246 | 65.0 | 1787 | 75 459 | 2.3 |
| Meningioma | 5.46 | 19 632 | 12.42 | 55 309 | 9.21 | 74 941 | 65.0 | 1577 | 73 364 | 2.1 |
| All other tumors of meninges# | 0.33 | 1275 | 0.25 | 1030 | 0.29 | 2305 | 49.0 | 210 | 2095 | 9.1 |
| Germ cell tumors and cysts | 0.06 | 235 | 0.02 | 97 | 0.04 | 332 | 28.0 | 229 | 103 | 69.0 |
| Tumors of sellar region | 3.46 | 13 098 | 3.75 | 15 398 | 3.56 | 28 496 | 52.0 | 117 | 28 379 | 0.4 |
| All other brain** | 1.42 | 5017 | 1.39 | 6363 | 1.40 | 11 380 | 69.0 | 4757 | 6623 | 41.8 |
| Lymphomas and hematopoietic neoplasms of the brain and ONS | 0.75 | 2764 | 0.54 | 2357 | 0.64 | 5121 | 64.0 | 5118 | †† | 99.9 |
NOS = not otherwise specified; ONS = other nervous system. Source: NAACCR Combined—National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program areas reported by NAACCR as meeting high-quality incidence data standards for 2003–2007 (46 states): Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming. The table excludes cancer cases that were identified as having invalid site/histology combinations.
The site grouping “Brain and ONS” includes cancer cases with primary sites C70.0–C72.9 and C75.1–C75.3. The category “Lymphomas and hematopoietic neoplasms of the brain and ONS” refers to those lymphomas and hematopoietic neoplasms with a primary site of C70.0–C72.9, C75.1–C75.3 as defined in Central Brain Tumor Registry of the United States (CBTRUS) (2010). CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006.
Benign and borderline cancer cases for the following tumors were recoded as malignant: diffuse astrocytoma, anaplastic astrocytoma, glioblastoma, oligodendroglioma, anaplastic oligodendroglioma, ependymoma/anaplastic ependymoma, mixed glioma, glioma malignant, NOS.
Incidence rates are per 100 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130).
Diffuse astrocytoma (as defined by World Health Organization 2007) includes the following histological groups: protoplasmic and fibrillary astrocytoma, astrocytoma NOS, and gemistocytic astrocytomas (9411).
All other tumors of neuroepithelial tissue includes the following histological groups: unique astrocytoma variants, ependymoma variants, choroid plexus, neuroepithelial, and pineal parenchymal, neuronal/glial, neuronal and mixed.
All other tumors of meninges includes the following histological groups: other mesenchymal, hemangioblastoma.
All other brain includes the following histological groups: chordoma/chondrosarcoma, hemangioma, unspecified neoplasms, and all other histologies which could not be classified above.
Counts less than six are not displayed except when equal to zero.
Table 6.
Age-standardized incidence rates and counts of pediatric (age 0–19 years) brain and other nervous system tumors including lymphomas by major histological groupings, sex, and behavior (nonmalignant, malignant), North American Association of Central Cancer Registries (NAACCR) combined, 2004–2007*
| Histological group† | Malignant, benign and borderline malignancy |
Malignant | Benign and borderline malignancy‡ | Percent malignant | ||||||
| Boys |
Girls |
Boys and girls |
||||||||
| Rate§ | Count | Rate§ | Count | Rate§ | Count | Median age | Count | Count | ||
| Brain and other nervous system | 48.80 | 7589 | 48.12 | 7147 | 48.47 | 14 736 | 10.0 | 9606 | 5130 | 65.2 |
| Tumors of neuroepithelial tissue | 35.71 | 5545 | 32.62 | 4834 | 34.20 | 10 379 | 8.0 | 8850 | 1529 | 85.3 |
| Pilocytic astrocytoma | 8.30 | 1284 | 7.90 | 1166 | 8.10 | 2450 | 9.0 | 2450 | 0 | 100.0 |
| Diffuse and anaplastic astrocytoma‖ | 3.73 | 580 | 3.40 | 504 | 3.57 | 1084 | 10.0 | 1084 | 0 | 100.0 |
| Glioblastoma | 1.60 | 249 | 1.19 | 175 | 1.40 | 424 | 12.0 | 424 | 0 | 100.0 |
| Oligodendroglioma and anaplastic oligodendroglioma | 0.83 | 129 | 0.77 | 115 | 0.80 | 244 | 14.0 | 244 | 0 | 100.0 |
| Mixed glioma | 0.32 | 50 | 0.40 | 60 | 0.36 | 110 | 13.0 | 110 | 0 | 100.0 |
| Glioma malignant, NOS | 5.40 | 833 | 5.89 | 869 | 5.64 | 1702 | 6.0 | 1702 | 0 | 100.0 |
| Embryonal/primitive/medulloblastoma | 5.67 | 880 | 4.44 | 661 | 5.07 | 1541 | 5.0 | 1541 | 0 | 100.0 |
| All other tumors of neuroepithelial tissue¶ | 9.87 | 1540 | 8.63 | 1284 | 9.27 | 2824 | 9.0 | 1295 | 1529 | 45.9 |
| Tumors of cranial and spinal nerves | 2.69 | 421 | 2.65 | 394 | 2.67 | 815 | 11.0 | 17 | 798 | 2.1 |
| Nerve sheath | 2.69 | 421 | 2.65 | 394 | 2.67 | 815 | 11.0 | 17 | 798 | 2.1 |
| Acoustic neuromas | 0.55 | 86 | 0.60 | 89 | 0.57 | 175 | 15.0 | †† | 174 | 0.6 |
| All other nerve sheath | 2.15 | 335 | 2.06 | 305 | 2.10 | 640 | 9.0 | 16 | 624 | 2.5 |
| Tumors of meninges | 1.72 | 270 | 1.89 | 283 | 1.80 | 553 | 14.0 | 64 | 489 | 11.6 |
| Meningioma | 1.20 | 188 | 1.27 | 189 | 1.23 | 377 | 14.0 | 24 | 353 | 6.4 |
| All other tumors of meninges# | 0.52 | 82 | 0.63 | 94 | 0.57 | 176 | 14.5 | 40 | 136 | 22.7 |
| Germ cell tumors and cysts | 2.64 | 413 | 1.14 | 170 | 1.91 | 583 | 12.0 | 501 | 82 | 85.9 |
| Tumors of sellar region | 3.64 | 567 | 7.64 | 1142 | 5.59 | 1709 | 15.0 | †† | 1706 | 0.2 |
| All other brain** | 2.39 | 373 | 2.18 | 324 | 2.29 | 697 | 11.0 | 171 | 526 | 24.5 |
| Lymphomas and hematopoietic neoplasms of the brain and ONS | 0.24 | 38 | 0.15 | 23 | 0.20 | 61 | 13.00 | 60 | †† | 98.4 |
NOS = not otherwise specified; ONS = other nervous system. Source: NAACCR Combined—National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program areas reported by NAACCR as meeting high-quality incidence data standards for 2003–2007 (46 states): Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming. The table excludes cancer cases that were identified as having invalid site/histology combinations.
The site grouping “Brain and ONS” includes cancer cases with primary sites C70.0–C72.9 and C75.1–C75.3. The category “Lymphomas and hematopoietic neoplasms of the brain and ONS” refers to those lymphomas and hematopoietic neoplasms with a primary site of C70.0–C72.9, C75.1–C75.3 as defined in Central Brain Tumor Registry of the United States (CBTRUS) (2010). CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006.
Benign and borderline cancer cases for the following tumors were recoded as malignant: diffuse astrocytoma, anaplastic astrocytoma, glioblastoma, oligodendroglioma, anaplastic oligodendroglioma, ependymoma/anaplastic ependymoma, mixed glioma, glioma malignant, NOS.
Incidence rates are per 1 000 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130).
Diffuse astrocytoma (as defined by World Health Organization 2007) includes the following histological groups: protoplasmic and fibrillary astrocytoma, astrocytoma NOS, and gemistocytic astrocytomas (9411).
All other tumors of neuroepithelial tissue includes the following histological groups: unique astrocytoma variants, ependymoma variants, choroid plexus, neuroepithelial, and pineal parenchymal, neuronal/glial, neuronal and mixed.
All other tumors of meninges includes the following histological groups: other mesenchymal, hemangioblastoma.
All other brain includes the following histological groups: chordoma/chondrosarcoma, hemangioma, unspecified neoplasms, and all other histologies which could not be classified above.
Counts less than six are not displayed except when equal to zero.
Incidence rates among children aged 0–19 years were much lower than in adults (48.47 per 1 000 000 children vs 24.55 per 100 000 adults), but the tumors were much more likely to be malignant in children: 65.2% vs 33.7% malignant in adults. Boys had only slightly more tumors of neuroepithelial tissue than girls (35.71 in boys vs 32.62 in girls per 1 000 000 children), yet adult men had incidence rates of neuroepithelial tumors 1.4 times higher than women. Tumors of the meninges were more likely to be malignant in children when compared with adults and occurred in boys and girls with similar frequency. Tumors of neuroepithelial tissue were more likely to be malignant in adults, whereas germ cell tumors were more likely to be malignant in children. Tumors of the nerve sheath were rarely malignant, and lymphomas of the brain were relatively rare in both adults and children.
Whites had the highest incidence rates of brain and ONS tumors (19.0 per 100 000 persons), followed by Hispanics (17.8 per 100 000 persons) and blacks (17.7 per 100 000 persons) (Table 7). AI/ANs had lower incidence rates of brain and ONS tumors (15.3 per 100 000 persons), and APIs had the lowest incidence rates (13.5 per 100 000 persons). Glioblastoma was the most common type of malignant brain tumor, with whites having the highest incidence rates, followed by Hispanics, blacks, and AI/ANs; with APIs having roughly one-half the rate of these tumors when compared with whites. The two most common types of nonmalignant brain tumors were higher among blacks compared with whites. Meningioma was the most common brain tumor, with black women having the highest incidence rates overall (9.7 per 100 000 persons), and black men having the highest incidence rates among men. Blacks and Hispanics had the highest incidence rates of tumors of the sellar region; however, acoustic neuromas occurred more than twice as often among whites compared with blacks.
Table 7.
Age-standardized rates and counts for tumors of the brain and other nervous system (nonmalignant and malignant), by histological grouping, race, and sex, North American Association of Central Cancer Registries (NAACCR) combined, 2004–2007*
| Sex | Histological group† | All races |
White |
Black |
API |
AI/AN CHSDA |
Hispanic |
Non-Hispanic |
|||||||
| Rate‡ | Count | Rate‡ | Count | Rate‡ | Count | Rate‡ | Count | Rate‡ | Count | Rate‡ | Count | Rate‡ | Count | ||
| Men and women | Brain and other nervous system | 18.9 | 213 525 | 19.0 | 181 790 | 17.7 | 20 158 | 13.5 | 6440 | 15.3 | 831 | 17.8 | 20 464 | 19.1 | 193 061 |
| Tumors of neuroepithelial tissue | 6.5 | 73 467 | 7.0 | 66 083 | 3.7 | 4573 | 3.2 | 1601 | 4.3 | 257 | 5.1 | 6613 | 6.7 | 66 854 | |
| Pilocytic astrocytoma | 0.3 | 3534 | 0.4 | 2989 | 0.2 | 332 | 0.2 | 85 | 0.4 | 31 | 0.2 | 502 | 0.3 | 3032 | |
| Diffuse and anaplastic astrocytoma§ | 1.0 | 10 851 | 1.1 | 9779 | 0.5 | 633 | 0.5 | 256 | 0.7 | 45 | 0.7 | 985 | 1.0 | 9866 | |
| Glioblastoma | 3.2 | 36 613 | 3.4 | 33 864 | 1.6 | 1798 | 1.4 | 634 | 1.8 | 86 | 2.4 | 2311 | 3.3 | 34 302 | |
| Oligodendroglioma and anaplastic oligodendroglioma | 0.4 | 4452 | 0.4 | 3995 | 0.2 | 237 | 0.2 | 122 | 0.3 | 15 | 0.3 | 452 | 0.4 | 4000 | |
| Mixed glioma | 0.2 | 2203 | 0.2 | 1978 | 0.1 | 115 | 0.1 | 67 | 0.2 | 12 | 0.2 | 245 | 0.2 | 1958 | |
| Glioma malignant, NOS | 0.4 | 4809 | 0.5 | 4125 | 0.3 | 426 | 0.3 | 134 | 0.3 | 22 | 0.4 | 573 | 0.4 | 4236 | |
| Embryonal/primitive/medulloblastoma | 0.2 | 2168 | 0.2 | 1813 | 0.1 | 226 | 0.1 | 77 | 0.2 | 15 | 0.2 | 441 | 0.2 | 1727 | |
| All other tumors of neuroepithelial tissue‖ | 0.8 | 8837 | 0.8 | 7540 | 0.6 | 806 | 0.4 | 226 | 0.4 | 31 | 0.6 | 1104 | 0.8 | 7733 | |
| Tumors of cranial and spinal nerves | 1.7 | 19 062 | 1.7 | 16 697 | 0.7 | 861 | 1.5 | 782 | 1.0 | 56 | 1.3 | 1535 | 1.7 | 17 527 | |
| Nerve sheath | 1.7 | 19 057 | 1.7 | 16 693 | 0.7 | 861 | 1.5 | 781 | 1.0 | 56 | 1.3 | 1535 | 1.7 | 17 522 | |
| Acoustic neuromas | 1.1 | 12 033 | 1.1 | 10 647 | 0.4 | 433 | 0.9 | 494 | 0.4 | 23 | 0.8 | 888 | 1.1 | 11 145 | |
| All other nerve sheath | 0.6 | 7024 | 0.6 | 6046 | 0.3 | 428 | 0.5 | 287 | 0.5 | 33 | 0.5 | 647 | 0.6 | 6377 | |
| Tumors of meninges | 6.8 | 77 799 | 6.7 | 65 175 | 7.8 | 8275 | 5.7 | 2551 | 5.9 | 279 | 6.9 | 6598 | 6.8 | 71 201 | |
| Meningioma | 6.6 | 75 318 | 6.4 | 63 063 | 7.6 | 8069 | 5.5 | 2447 | 5.7 | 266 | 6.7 | 6298 | 6.6 | 69 020 | |
| All other tumors of meninges¶ | 0.2 | 2481 | 0.2 | 2112 | 0.2 | 206 | 0.2 | 104 | 0.2 | 13 | 0.2 | 300 | 0.2 | 2181 | |
| Germ cell tumors and cysts | 0.1 | 915 | 0.1 | 736 | 0.1 | 84 | 0.1 | 63 | 0.1 | 6 | 0.1 | 188 | 0.1 | 727 | |
| Tumors of sellar region | 2.7 | 30 205 | 2.4 | 22 836 | 4.4 | 5144 | 2.3 | 1162 | 3.0 | 180 | 3.3 | 4244 | 2.6 | 25 961 | |
| All other brain# | 1.1 | 12 077 | 1.1 | 10 263 | 1.1 | 1221 | 0.7 | 281 | 1.0 | 53 | 1.2 | 1286 | 1.0 | 10 791 | |
| Lymphomas and hematopoietic neoplasms of the brain and ONS | 0.5 | 5182 | 0.5 | 4410 | 0.4 | 504 | 0.4 | 203 | 0.4 | 20 | 0.5 | 545 | 0.5 | 4637 | |
| Men | Brain and other nervous system | 17.3 | 90 870 | 17.6 | 78 476 | 15.5 | 7694 | 11.6 | 2583 | 12.8 | 328 | 15.1 | 8445 | 17.7 | 82 425 |
| Tumors of neuroepithelial tissue | 7.7 | 40 820 | 8.2 | 36 917 | 4.2 | 2342 | 3.8 | 883 | 4.9 | 141 | 5.8 | 3620 | 8.0 | 37 200 | |
| Pilocytic astrocytoma | 0.3 | 1834 | 0.4 | 1562 | 0.2 | 158 | 0.2 | 43 | 0.4 | 14 | 0.2 | 265 | 0.4 | 1569 | |
| Diffuse and anaplastic astrocytoma§ | 1.1 | 6013 | 1.2 | 5479 | 0.5 | 309 | 0.6 | 134 | 0.6 | 18 | 0.8 | 540 | 1.2 | 5473 | |
| Glioblastoma | 4.0 | 20 841 | 4.3 | 19 330 | 2.0 | 958 | 1.8 | 375 | 2.1 | 50 | 2.9 | 1293 | 4.1 | 19 548 | |
| Oligodendroglioma and anaplastic oligodendroglioma | 0.5 | 2467 | 0.5 | 2197 | 0.2 | 135 | 0.3 | 74 | 0.3 | 9 | 0.3 | 224 | 0.5 | 2243 | |
| Mixed glioma | 0.2 | 1277 | 0.3 | 1147 | 0.1 | 65 | 0.1 | 37 | 0.2 | 6 | 0.2 | 139 | 0.2 | 1138 | |
| Glioma malignant, NOS | 0.5 | 2462 | 0.5 | 2152 | 0.3 | 192 | 0.2 | 60 | 0.3 | 12 | 0.4 | 293 | 0.5 | 2169 | |
| Embryonal/primitive/medulloblastoma | 0.2 | 1233 | 0.2 | 1032 | 0.2 | 122 | 0.2 | 44 | 0.3 | 11 | 0.2 | 269 | 0.2 | 964 | |
| All other tumors of neuroepithelial tissue‖ | 0.9 | 4693 | 0.9 | 4018 | 0.6 | 403 | 0.4 | 116 | 0.7 | 21 | 0.7 | 597 | 0.9 | 4096 | |
| Tumors of cranial and spinal nerves | 1.7 | 9170 | 1.7 | 8044 | 0.7 | 395 | 1.5 | 359 | 1.0 | 28 | 1.2 | 702 | 1.8 | 8468 | |
| Nerve sheath | 1.7 | 9168 | 1.7 | 8042 | 0.7 | 395 | 1.5 | 359 | 1.0 | 28 | 1.2 | 702 | 1.8 | 8466 | |
| Acoustic neuromas | 1.0 | 5704 | 1.1 | 5064 | 0.3 | 187 | 0.9 | 221 | 0.4 | 10 | 0.7 | 395 | 1.1 | 5309 | |
| All other nerve sheath | 0.6 | 3464 | 0.7 | 2978 | 0.3 | 208 | 0.5 | 138 | 0.6 | 18 | 0.5 | 307 | 0.7 | 3157 | |
| Tumors of meninges | 4.2 | 21 177 | 4.1 | 17 823 | 5.0 | 2205 | 3.3 | 663 | 3.6 | 73 | 3.8 | 1689 | 4.2 | 19 488 | |
| Meningioma | 3.9 | 19 820 | 3.8 | 16 661 | 4.8 | 2107 | 3.1 | 602 | 3.4 | 68 | 3.6 | 1529 | 4.0 | 18 291 | |
| All other tumors of meninges¶ | 0.2 | 1357 | 0.3 | 1162 | 0.2 | 98 | 0.2 | 61 | ** | ** | 0.2 | 160 | 0.3 | 1197 | |
| Germ cell tumors and cysts | 0.1 | 648 | 0.1 | 526 | 0.1 | 51 | 0.2 | 45 | ** | ** | 0.1 | 146 | 0.1 | 502 | |
| Tumors of sellar region | 2.6 | 13 665 | 2.3 | 10 555 | 4.4 | 2166 | 2.3 | 524 | 2.6 | 65 | 3.0 | 1677 | 2.5 | 11 988 | |
| All other brain# | 1.1 | 5390 | 1.1 | 4611 | 1.2 | 535 | 0.6 | 109 | 0.6 | 17 | 1.2 | 611 | 1.1 | 4779 | |
| Lymphomas and hematopoietic neoplasms of the brain and ONS | 0.5 | 2802 | 0.5 | 2379 | 0.5 | 279 | 0.5 | 105 | 0.5 | 13 | 0.6 | 340 | 0.5 | 2462 | |
| Women | Brain and other nervous system | 20.3 | 122 655 | 20.3 | 103 314 | 19.5 | 12 464 | 15.0 | 3857 | 17.6 | 503 | 20.4 | 12 019 | 20.4 | 110 636 |
| Tumors of neuroepithelial tissue | 5.6 | 32 647 | 5.9 | 29 166 | 3.3 | 2231 | 2.7 | 718 | 3.7 | 116 | 4.5 | 2993 | 5.7 | 29 654 | |
| Pilocytic astrocytoma | 0.3 | 1700 | 0.3 | 1427 | 0.2 | 174 | 0.2 | 42 | 0.4 | 17 | 0.2 | 237 | 0.3 | 1463 | |
| Diffuse and anaplastic astrocytoma§ | 0.8 | 4838 | 0.9 | 4300 | 0.5 | 324 | 0.4 | 122 | 0.8 | 27 | 0.7 | 445 | 0.9 | 4393 | |
| Glioblastoma | 2.5 | 15 772 | 2.7 | 14 534 | 1.4 | 840 | 1.0 | 259 | 1.5 | 36 | 2.0 | 1018 | 2.6 | 14 754 | |
| Oligodendroglioma and anaplastic oligodendroglioma | 0.4 | 1985 | 0.4 | 1798 | 0.1 | 102 | 0.2 | 48 | 0.2 | 6 | 0.3 | 228 | 0.4 | 1757 | |
| Mixed glioma | 0.2 | 926 | 0.2 | 831 | 0.1 | 50 | 0.1 | 30 | 0.2 | 6 | 0.1 | 106 | 0.2 | 820 | |
| Glioma malignant, NOS | 0.4 | 2347 | 0.4 | 1973 | 0.3 | 234 | 0.3 | 74 | 0.3 | 10 | 0.3 | 280 | 0.4 | 2067 | |
| Embryonal/primitive/medulloblastoma | 0.2 | 935 | 0.2 | 781 | 0.1 | 104 | 0.1 | 33 | ** | ** | 0.2 | 172 | 0.2 | 763 | |
| All other tumors of neuroepithelial tissue‖ | 0.7 | 4144 | 0.8 | 3522 | 0.5 | 403 | 0.4 | 110 | 0.2 | 10 | 0.6 | 507 | 0.8 | 3637 | |
| Tumors of cranial and spinal nerves | 1.7 | 9892 | 1.8 | 8653 | 0.7 | 466 | 1.5 | 423 | 0.9 | 28 | 1.4 | 833 | 1.7 | 9059 | |
| Nerve sheath | 1.7 | 9889 | 1.8 | 8651 | 0.7 | 466 | 1.5 | 422 | 0.9 | 28 | 1.4 | 833 | 1.7 | 9056 | |
| Acoustic neuromas | 1.1 | 6329 | 1.1 | 5583 | 0.4 | 246 | 1.0 | 273 | 0.5 | 13 | 0.8 | 493 | 1.1 | 5836 | |
| All other nerve sheath | 0.6 | 3560 | 0.6 | 3068 | 0.3 | 220 | 0.5 | 149 | 0.5 | 15 | 0.5 | 340 | 0.6 | 3220 | |
| Tumors of meninges | 9.1 | 56 622 | 8.9 | 47 352 | 9.9 | 6070 | 7.6 | 1888 | 7.9 | 206 | 9.5 | 4909 | 9.1 | 51 713 | |
| Meningioma | 8.9 | 55 498 | 8.7 | 46 402 | 9.7 | 5962 | 7.5 | 1845 | 7.7 | 198 | 9.3 | 4769 | 8.9 | 50 729 | |
| All other tumors of meninges¶ | 0.2 | 1124 | 0.2 | 950 | 0.2 | 108 | 0.2 | 43 | 0.2 | 8 | 0.2 | 140 | 0.2 | 984 | |
| Germ cell tumors and cysts | 0.1 | 267 | 0.0 | 210 | 0.0 | 33 | 0.1 | 18 | ** | ** | 0.1 | 42 | 0.1 | 225 | |
| Tumors of sellar region | 2.9 | 16 540 | 2.6 | 12 281 | 4.5 | 2978 | 2.3 | 638 | 3.5 | 115 | 3.7 | 2567 | 2.8 | 13 973 | |
| All other brain# | 1.1 | 6687 | 1.0 | 5652 | 1.1 | 686 | 0.7 | 172 | 1.4 | 36 | 1.2 | 675 | 1.0 | 6012 | |
| Lymphomas and hematopoietic neoplasms of the brain and ONS | 0.4 | 2380 | 0.4 | 2031 | 0.3 | 225 | 0.4 | 98 | 0.3 | 7 | 0.4 | 205 | 0.4 | 2175 | |
AI/AN = American Indian/Alaska Native; API = Asian/Pacific Islander; CHSDA = Contract Health Services Delivery Area; IHS = Indian Health Service; NOS = not otherwise specified; ONS = other nervous system. Source: NAACCR Combined—National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program areas reported by NAACCR as meeting high-quality incidence data standards for 2003–2007 (46 states): Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming. The table excludes cancer cases that were identified as having invalid site/histology combinations.
The site grouping “Brain and ONS” includes cancer cases with primary sites C70.0–C72.9 and C75.1–C75.3. The category “Lymphomas and hematopoietic neoplasms of the brain and ONS” refers to those lymphomas and hematopoietic neoplasms with a primary site of C70.0–C72.9 and C75.1–C75.3 as defined in Central Brain Tumor Registry of the United States (CBTRUS) (2010). CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006.
Incidence rates are per 100 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130).
Diffuse astrocytoma (as defined by World Health Organization 2007) includes the following histological groups: protoplasmic and fibrillary astrocytoma, astrocytoma NOS, and gemistocytic astrocytomas (9411).
All other tumors of neuroepithelial tissue includes the following histological groups: unique astrocytoma variants, ependymoma variants, choroid plexus, neuroepithelial, and pineal parenchymal, nonmalignant and malignant neuronal/glial, neuronal and mixed.
All other tumors of meninges include the following histological groups: other mesenchymal, hemangioblastoma.
All other brain includes the following histological groups: chordoma/chondrosarcoma, hemangioma, unspecified neoplasms, and all other histologies which could not be classified above.
Statistic not displayed because less than six cancer cases in this category.
Childhood brain and ONS tumor counts and incidence rates using ICCC-3 definitions are presented in Table 8. This classification system is used widely with childhood brain and ONS tumors but does not allow for comparison with adults. Childhood cancer incidence rates presented in Table 8 may differ from those presented elsewhere in this article because of the different classification systems used to produce the tables.
Table 8.
Age-standardized and age-specific incidence rates for pediatric brain and other nervous system tumors including lymphomas (primary sites C70.0–C72.9, C75.1–C75.3; nonmalignant and malignant), by International Classification of Childhood Cancer (ICCC) men and women combined, North American Association of Central Cancer Registries (NAACCR) combined, 2004–2007*
| ICCC category | Men and women |
|||||||||||||
| Age-standardized rates and counts†, age 0–14 y |
Age-standardized rates and counts†,age 0–19 y |
Age-specific rates and counts‡ |
||||||||||||
| Rate | Count | Rate | Count | Age < 1 y |
Age 1–4 y |
Age 5–9 y |
Age 10–14 y |
Age 15–19 y |
||||||
| Rate | Count | Rate | Count | Rate | Count | Rate | Count | Rate | Count | |||||
| II Lymphomas and reticuloendothelial neoplasms | 0.15 | 34 | 0.19 | 59 | § | ‖ | § | 8 | § | 8 | § | 15 | 0.32 | 25 |
| III CNS and misc intracranial and intraspinal neoplasms | 41.08 | 9258 | 41.44 | 12 587 | 42.50 | 654 | 47.09 | 2829 | 40.54 | 2951 | 36.80 | 2824 | 42.50 | 3329 |
| III (a) Ependymomas and choroid plexus tumors | 4.02 | 916 | 3.78 | 1158 | 9.49 | 146 | 5.93 | 356 | 3.02 | 220 | 2.53 | 194 | 3.09 | 242 |
| III (b) Astrocytomas | 16.70 | 3760 | 15.78 | 4784 | 12.48 | 192 | 19.79 | 1189 | 16.32 | 1188 | 15.52 | 1191 | 13.07 | 1024 |
| III (c) Intracranial and intraspinal embryonal tumors | 5.95 | 1350 | 4.97 | 1509 | 9.42 | 145 | 9.35 | 562 | 5.62 | 409 | 3.05 | 234 | 2.03 | 159 |
| III (d) Other gliomas | 5.58 | 1249 | 5.22 | 1573 | 2.53 | 39 | 5.74 | 345 | 7.10 | 517 | 4.54 | 348 | 4.14 | 324 |
| III (e) Other specified intracranial/intraspinal neoplasms | 7.37 | 1653 | 10.07 | 3072 | 6.17 | 95 | 4.96 | 298 | 7.09 | 516 | 9.70 | 744 | 18.11 | 1419 |
| III (f) Unspecified intracranial and intraspinal neoplasms | 1.46 | 330 | 1.61 | 491 | 2.40 | 37 | 1.31 | 79 | 1.39 | 101 | 1.47 | 113 | 2.06 | 161 |
| IV Neuroblastomas and other peripheral nervous cell tumors | 0.61 | 142 | 0.51 | 159 | 2.73 | 42 | 1.13 | 68 | § | 15 | 0.22 | 17 | 0.22 | 17 |
| IX Soft tissue and other extraosseous sarcomas | 3.25 | 733 | 3.81 | 1164 | 4.94 | 76 | 3.01 | 181 | 2.72 | 198 | 3.62 | 278 | 5.50 | 431 |
| X Germ cell and trophoblastic tumors and neoplasms of gonads | 1.68 | 379 | 1.91 | 583 | 3.70 | 57 | 0.47 | 28 | 1.09 | 79 | 2.80 | 215 | 2.60 | 204 |
| All other categories¶ | 0.49 | 110 | 0.80 | 245 | § | 9 | 0.35 | 21 | 0.37 | 27 | 0.69 | 53 | 1.72 | 135 |
Source: NAACCR Combined— National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program areas reported by NAACCR as meeting high-quality incidence data standards for 2003–2007 (46 states): Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming. The table excludes cancer cases that were identified as having invalid site/histology combinations.
Incidence rates are per 1 000 000 persons and were age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, 10–14, … , 80–84, ≥85 years, Census P25–1130).
Incidence rates are per 1 000 000 persons.
Statistic not displayed because less than six cancer cases in this category.
Counts in specific age groups of less than six cancer cases are not displayed.
Includes ICCC groupings: I, V, VI, VII, VIII, XI, XII, not classified.
Childhood brain and ONS tumors demonstrate unique age-specific incidence patterns by sex and type of tumor classification (Supplementary Table 3, available online). The incidence rate remained below six per 100 000 persons until age 25–29 years, after which the incidence increased steadily until age 84 years. By sex, incidence rates were higher in boys through age 10–14 years, after which the incidence rates became higher in women, primarily because of the large increase in meningiomas in this group (Supplementary Tables 4 and 5, available online). Incidence rates were higher in men or similar to women for all age groups for most tumors of neuroepithelial tissue, nerve sheath tumors, germ cell tumors, and lymphomas; however, women had a much higher incidence rate of meningioma than men, beginning with age group 20–24 years. Incidence rates for tumors of the sellar region were higher in women until age 45–49 years; however, this pattern was reversed at 50 years and older. Acoustic neuromas began increasing from age 40 years, peaked at ages 65–69 years, after which the incidence rates decreased.
Trends of malignant neuroepithelial tumors by histological group are shown in Figure 1 and Supplementary Table 6 (available online). The incidence of these tumors in men and women combined increased at a rate of 1.9% per year from 1980 to 1987 and decreased at a rate of 0.4% per year from 1987 to 2007, resulting in a minimal net change from 1980 to 2007. However, trends in incidence rates differed markedly among histological groups within this category. Marked changes in incidence rates for primary brain lymphomas also were observed (data not shown).
Figure 1.
Trends in malignant neuroepithelial tumors in men and women by histology, 1980–2007. Trends calculated using joinpoint analysis with up to four joinpoints on Surveillance, Epidemiology, and End Results 9 registry data (Connecticut, Hawaii, Iowa, New Mexico, Utah; metropolitan areas of San Francisco, Detroit, Atlanta, Seattle-Puget Sound).
Relative survival (14) for brain and ONS tumors is strongly related to age at diagnosis, histological type, and era of diagnosis, as demonstrated in Figure 2. Five-year survival for all malignant tumors of neuroepithelial tissue as well as for most histological types and age groups has increased over time. Five-year survival among children and adolescents with all malignant tumors of neuroepithelial tissue combined increased from 62.9% for those diagnosed in 1980–1989 to 75.3% in 2000–2006 (Supplementary Table 7, available online). Favorable survival and trends also were observed for those aged 20–39 years, for whom 5-year survival increased from 54.1% in 1980–1989 to 65.1% in 2000–2006. However, among those diagnosed at age 40–64 years, survival increased from only 16.1% to 26.6%; among those diagnosed at age 65 years or older, the 5-year survival was under 5%, even in the most recent period (2003–2007). Among the most common tumors, the 5-year survival for pilocytic astrocytoma increased from 90.1% in 1980–1989 to 96.4% in 2000–2006 among children aged 0–19 years. Survival was nearly as high in those aged 20–39 years and increased markedly in those aged 40 years or older, from 46.1% in 1980–1989 to 83.5% in 2000–2006. In contrast, there was relatively little improvement in survival for astrocytomas during this time interval. Irregular trends and low survival for glioblastoma were observed in the two youngest age groups (0–19 and 20–39 years), with 5-year survival exceeding 20% only in the most recent time period. In the two older age groups (40–64 and ≥65 years), glioblastoma survival exceeded 5% at 5 years only in those aged 40–64 years diagnosed in 2000–2006. Five-year survival for oligodendroglioma and anaplastic oligodendroglioma in those aged 0–19 years increased from 70.2% to 90.8% in 2000–2006. Survival of greater than 75% was observed for those aged 20–39 years, in each time period. Although survival increased over time for those aged 40–64 years and those 65 years and older, it remained generally lower than in the younger age groups. In contrast, survival trends for neuroepithelial tumors in the grouping “embryonal, primitive, and medulloblastoma” differed by age group, with marked increases for those in the 0–19 and 20–39 year age ranges but marked decreases for those aged 40 years and older. Survival for malignant gliomas, not otherwise specified, showed greater improvement over time than for mixed gliomas.
Figure 2.
Five-year relative survival for neuroepithelial histologies (malignant) in men and women by age and time period. Relative survival calculated based on histology, age at diagnosis, and decade of diagnosis using data from Surveillance, Epidemiology, and End Results 9 registries (Connecticut, Hawaii, Iowa, New Mexico, Utah; metropolitan areas of San Francisco, Detroit, Atlanta, Seattle-Puget Sound).
Death rates for malignant brain tumors during 1999–2007 were stable in children (aged 0–19 years) but decreased in adults (aged ≥20 years) at a rate of 1.2% per year. Death rates for benign brain tumors decreased in both children (−2.5% per year) and adults (−2.2% per year). Trends in mortality for malignant vs non-malignant brain tumors could not be examined for earlier time periods because of inconsistencies in ICD coding over time.
Discussion
This “Annual Report to the Nation” is the first to document a statistically significant decrease in lung cancer incidence and death rates among women from 2003 to 2007 (Tables 1 and 2), more than a decade after the rates began to decrease in men. Although cigarette smoking peaked in men who served in World War II and were born in the early 1920s, it peaked in women born in the late 1930s (32,33). The decrease in lung cancer rates in women that we are seeing now reflects the later uptake of cigarette smoking among women. The decrease in lung cancer rates in women can be expected to continue for at least two decades as women in the older generations with higher lung cancer risk are replaced by the subsequent younger generations with lower risk. But trends may be interrupted as women born around 1960, who have higher lung cancer and smoking rates, enter the high-risk age groups (11,34,35). In contrast to women, lung cancer rates in men are expected to continue to decrease in the subsequent younger generations (11).
In addition to lung cancer, death rates in the most recent period (2003–2007) showed a statistically significant decrease for seven of the remaining 14 leading cancers among both men and women (cancers of the colorectum, kidney, stomach, brain, leukemia, non-Hodgkin lymphoma, and myeloma) as well as for prostate and oral cancer among men and cancers of the breast, ovary, and bladder among women. As a result, death rates from all cancers combined continued to decrease among both men and women and in all racial and ethnic groups, except among AI/AN women. These decreases indicate real progress in cancer control, reflecting a combination of primary prevention, early detection, and treatment (2–12). However, death rates continued to increase for cancers of the pancreas and liver among men and women, and for uterine cancer in women, cancers for which there are no established screening tests. Among men, death rates also increased for melanoma, for which population screening is not recommended but prevention and early detection strategies are available (36,37). Among children, long-term (1975–2007) trends in death rates continued to decrease, although at a slower pace during the recent decade than in earlier years.
The overall cancer incidence rates showed a statistically significant decrease during the most recent period (2003–2007) in women, but the decrease was not statistically significant in men. These trends are driven largely by trends in the most common cancer sites (lung, colorectal, prostate, and female breast), accounting for more than 50% of the overall rates in both men and women (38). Incidence rates decreased for cancers of the lung, colorectum, and oral cavity in both men and women; for breast, cervix, uterine, and bladder cancers in women; and for stomach and brain cancers in men. In contrast, incidence rates increased for kidney and pancreatic cancers and melanoma in both men and women, for liver cancer in men, and for thyroid cancer and leukemia in women. Prostate cancer rates showed a non-statistically significant increase. Factors that contribute to these trends were discussed previously (4,7,8,11,12,34,35) and include changes in risk factors, screening modalities, and diagnostic practices.
Of the leading cancers, prostate cancer and breast cancer are of special note because they are the most frequently diagnosed cancers and second leading cause of cancer death among men and women, respectively. Prostate cancer incidence has fluctuated through the years, decreasing during 1992–1995, increasing during 1995–2001, decreasing during 2001–2005, and increasing again during 2005–2007, albeit non-statistically significantly (Table 1). Prostate cancer death rates have decreased substantially over time (39), but the contribution of prostate-specific antigen screening to this decrease and the risks and benefits for individual men remain uncertain (40–44).
Trends in breast cancer incidence over time reflect long-term changes in reproductive and other risk factors, introduction and prevalence of mammography screening, and use of hormones among postmenopausal women (45) (Table 1 and Supplementary Table 1, available online). Breast cancer incidence rates stabilized from 2003 to 2007 (46) after decreasing sharply between 2002 and 2003, which was temporally associated with the dramatic decrease in the use of postmenopausal hormonal replacement therapy (47,48). The stabilization of the rates after the sharp decrease between 2002 and 2003 may in part reflect the role of hormonal replacement therapy as a promoting agent rather than as an initiating agent in the development of breast cancer (DeSantis et al., unpublished data). Meanwhile, breast cancer death rates continued to exhibit a statistically significant decrease. Mammography screening generally is accepted to reduce breast cancer mortality and has been recommended for some time, although recommendations have varied among organizations with respect to age at initiation for average risk women, screening intervals, and screening modalities, especially for high-risk women (49–55).
Of concern is the long-term increase in cancer incidence rates among children, which may be because of larger increases in incidence rates for the lymphoid leukemias and proportionately smaller increases for other childhood cancers (56). Considerable progress has occurred for many types of childhood cancers, resulting in decreases in cancer death rates among children since 1975, although the rate of decrease has slowed since the mid-1990s. These decreases have resulted from refinements in treatment that substantially improved survival for many childhood cancers. However, for some types of childhood cancer, including some brain tumors, progress has been more modest and current treatments remain inadequate (56).
Differences in rates and trends in incidence and death rates for specific cancers for different racial and ethnic groups and for men and women suggest differences in risk behaviors, socioeconomic status, and access to and use of screening and treatment (57,58). It is particularly important to monitor these trends to identify opportunities and set priorities for cancer control interventions. Where possible, it is important to examine multiple indices and risk indicators at the national, state, and local level. In addition, although not always feasible in national reports, it is important to recognize that categorizing the population by broad racial and ethnic categories may mask important differences within and among populations.
We provided a comprehensive evaluation of the incidence and mortality for all primary (malignant and nonmalignant) brain and ONS tumors, as well as trends in incidence and survival on a national level. This report expands on the descriptive epidemiology of primary brain tumors presented by the Central Brain Tumor Registry of the United States in its annual statistical report (16). Collection of nonmalignant (benign or uncertain behavior) tumors began nationwide in diagnosis year 2004, allowing for 4 years of data (2004–2007) to be presented here. Nonmalignant tumors accounted for the majority of all brain tumors, representing two-thirds of all adult and one-third of all childhood (aged 0–19 years) brain tumors. Capturing surveillance data on nonmalignant brain tumors has demonstrated that meningioma is the most common form of brain tumor in the United States. Differing patterns by race, sex, and age were seen for different types of malignant and nonmalignant brain tumors. Although the reasons for these differences have not been elucidated, they may prove important for discovering differences in the etiology of these diverse tumors.
An important finding of the current analysis is the relative stability of the long-term incidence trends of malignant tumors of the neuroepithelial tissue. During the 27-year (1980–2007) time period studied, an increase of 1.9% per year during 1980–1987 was counterbalanced by a decrease of 0.4% per year during the remaining 20 years, resulting in nearly identical incidence rates at the beginning and end of the study. However, marked differences in trends were observed for histological groups within this category of tumors. As with many cancers, trends may be influenced by a number of factors, including changes in diagnostic techniques and changes in coding and classification. The introduction of computed tomography scans in the 1970s and magnetic resonance imaging scans and stereotactic biopsy in the mid-1980s (59) has led to less invasive methods for diagnosing these tumors and contributed in part to fluctuations in the incidence rates over time. Revisions in the World Health Organization’s histological classification of ONS tumors and the ICD-O also occurred during the period of the study, along with changes in the multiple primary rules for malignant brain tumors and the introduction of multiple primary rules for nonmalignant brain tumors. Brain and ONS tumors have been particularly difficult to diagnose pathologically because they often are heterogeneous histologically, genetically, and therapeutically (17,60). However, progress in understanding the molecular pathogenesis of malignant gliomas has begun to allow for better classification of these tumors (61–63).
In contrast to tumors of neuroepithelial tissue, marked changes in the incidence of lymphomas of the brain have been observed, likely because of increases in AIDS-related lymphomas in the 1980s, followed by decreases in AIDS-related lymphomas after the introduction of highly active antiretroviral therapy in the 1990s (64). The short time period for which data on nonmalignant brain tumors are available in the United States precluded analysis of temporal trends.
Modest improvements in survival for many types of brain and ONS tumors likely result from improvements in diagnostic and surgical techniques, radiotherapy, chemotherapy, biological therapy, and the use of multimodality therapy (65,66). Despite improvements in treatment, major prognostic factors include the histology of the tumor, whether complete surgical resection is achieved, and the age of the patient at diagnosis (66). Late effects of therapy for childhood brain tumors are substantial and include neurocognitive deficiencies, hormone deficits, growth impairment, second primary brain tumors, and ototoxicity related to platinum chemotherapy (67–69).
Several reviews of risk factors for brain tumors have been published recently (70–78). The relatively low variation in incidence and death rates for cancer of the brain and ONS nationally and internationally suggests that environmental risk factors do not play a major role in this disease (70–74). In fact, other than hereditary tumor syndromes (17) and increased familial risk without a known syndrome (79–82), the only known modifiable causal risk factor for brain tumors is exposure to ionizing radiation (71–74,78). Variability in age at onset and molecular tumor characteristics suggests that risk factors for brain tumors may differ by histological type (16,17,75,75–77). An example is the mostly consistent inverse association that has been observed between history of atopic disease, including allergies and asthma, and risk of glioma (72–74,83–97) and possibly meningioma (78,95–98); but no association with nerve sheath tumors has been found (84).
Several reviews summarize studies evaluating exposure to cellular phones and the risk of brain tumors (78,99–102). Short-term (<10 years) exposures to cellular telephones appear to have no association with risk of brain tumors. However, the association with long-term (>10 years) use remains unclear, primarily because of the relatively recent adoption of widespread use of cellular phones, as well as issues of bias and study design. Acoustic neuromas are of particular interest with regard to cellular phone use because of the proximity of these tumors to the phone. However, studies that have examined this association have mixed results and limited numbers of long-term users; further studies with longer term follow-up will be needed to evaluate whether there is an increased risk of acoustic neuromas associated with the use of cellular phones (99–102). A recent study using data from SEER 9 registries for 1977–2006 found decreasing or stable brain cancer incidence rate trends for whites in most age groups except among women aged 20–29 years in 1992–2006, which was driven by a rising incidence of frontal lobe cancers (103). We examined age- and sex-specific trends in overall malignant brain cancer incidence rates among whites in the SEER 13 registries from 1992 to 2007 and NAACCR data for 1995–2007 (Supplementary Table 8, available online). Although the short time period for which non-malignant data are available in the United States precludes analysis of temporal trends, the relatively large number of acoustic neuromas identified in the first 4 years of data collection suggests that etiologic studies will be possible in the future.
Limitations
High-quality cancer surveillance data now cover 93% of the US population for incidence and the entire population for mortality; however, certain limitations in data sources, data collection, and analyses may have influenced the findings of this report. First, state and national population estimates are provided annually by the Census Bureau to estimate intercensal populations. Differences between the numerator (incidence data) and denominator (US Census population data) can occur in the designation of race and/or ethnicity, place of residency, age, single vs multiple races, and the like. Every effort is made to ensure that the definition of the numerator and denominator are the same. Intercensal population estimates based on numbers updated by birth and death data are more subject to error than the estimates based on the actual count. Although these population estimates are believed to be the most accurate available, errors in the estimates may increase as time passes from the original recording of Census data. The NCI developed modifications to these Census estimates to attempt to account for changes in 2005 county-level populations because of displacement of people after Hurricanes Katrina and Rita in the most-affected counties of Louisiana, Mississippi, Alabama, and Texas. Censal and other data are used to classify the incidence cases, and census definitions are used to determine residency for the incidence cases. Race and ethnicity, however, generally are self-reported, but for the incidence cases, this information may come from a wider group of sources (patient, relative, nurse, doctor, coroner, funeral director). To enhance race and ethnicity, determination for the incidence cases, special studies and algorithms are used. For example, a match of incidence cases to IHS rosters is undertaken to correct the possible underreporting of AI/ANs, and NAACCR has developed guidelines and algorithms for enhancing Hispanic-Latino and API identification. Consistency over time in definitions for both census and incidence data is an issue, and efforts have been made to bridge single race and multiple race reporting (more information available on http://www.cdc.gov/nchs/nvss/bridged_race.htm). Second, joinpoint models were used to describe long-term (1992–2007) and short-term (1998–2007) trends. The AAPC, a summary measure of a trend over a prespecified fixed interval based on an underlying joinpoint model, was used to describe all trend data. The joinpoint model is preferable to single linear regression when a sufficient number of years are available for analysis because it enables identification of recent changes in magnitude and direction of trends. However, it may mask the underlying data and give an impression of a continuous increase or decrease over time when this is not the case. In addition, although methods have been adapted recently to adjust for delayed reporting of aggregated data similar to earlier published methods used for incidence from the nine oldest SEER registries (30), methods have not been tested on data from registries outside of SEER and were employed in our analysis only for SEER 9 and SEER 13. Delayed reporting may affect the most recent joinpoint segments, overestimating recent decreases and underestimating recent increases.
Third, US Department of Veterans Affairs (VA) hospitals traditionally have been a major source of data for cancers diagnosed among veterans, representing approximately 3%–8% of cancer diagnoses among men. A 2007 policy change regarding the transfer of VA cancer data to state central cancer registries has resulted in incomplete reporting of VA hospital cases in some but not all state registries. This change has affected reporting from the third quarter of the 2004 diagnosis year through the current time period. As a result, cancer incidence rates among men for 2005–2007 are thought to be underestimated by 0.8%–2% for all cancers combined, according to independent statistical analyses conducted by the CDC and SEER. The level of underreporting varied from 0.5% to 4% according to cancer site, race, and age group (14,104). The amount of underestimation also may vary by local VA facility reporting patterns and the VA’s contribution to the total number of cancers. Progress in collecting VA data has been made in many states with the enactment of special data-sharing agreements with the VA. Over time, as cancer registries receive these missing VA cases, national cancer incidence estimates will be more complete and accurate.
Fourth, as routinely noted in the Annual Reports to the Nation (1–12), the broad racial and ethnic groups categorized for our analyses may mask variations in the cancer burden by country of origin; for example, Chinese and Vietnamese in the API group (105) and Cubans and Mexicans in the Hispanic group (9,106), or by other unique characteristics of high- or low-risk populations (107–110). Also, cancer rates for populations may be limited by difficulties in ascertaining race and ethnicity information from medical records, death certificates, and census reports (25).
Future Directions
The observed decreases in overall cancer incidence and death rates in nearly all racial and ethnic groups are highly encouraging. This progress could be accelerated by comprehensively applying existing cancer control knowledge of cancer prevention, early detection, and treatment to public health and clinical practices. Unfortunately, at this point in time, not all cancer sites are amenable to cancer control practices, and innovative methods to study these cancers and rare tumors must be developed. For example, the relative rarity of brain tumors, including many histological subtypes, has required investigators to establish consortia and pooled studies, especially for studies of genetic risk factors and gene–environment interaction (70,111–114). Many advances are being made in the molecular characterization of brain and ONS tumors and many other types of cancer. Tumor biospecimen banking linked with treatment and outcome information will be particularly important in studying the prognostic and predictive value of such markers and in developing targeted therapies (115–117) to improve effectiveness, lessen toxicity, and measure response to therapy more quickly. It is too early to assess the impact of some treatment advances or the progress in targeted therapy that is expected to emerge in future years.
The US population aged 65 years and older is expected to double in size by 2030 (about 71 million persons) compared with the number reported in the 2000 census (118). Improvements in health and welfare also mean that individuals are expected to live longer, often with a range of health conditions that include the diagnosis of cancer. Even with declining cancer incidence rates, the absolute number of individuals diagnosed with cancer will continue to increase because of these population changes, leading to increased demand for cancer-related medical services through the spectrum of diagnosis, active treatment, and posttreatment medical management. Effective management of the cancer burden will require the application of sound cancer control strategies in prevention, detection, treatment, and survivorship, as well as resources to provide good quality of care. Continued utilization of quality population-based data systems and translation of evidence-based clinical and basic research findings to public health practices are essential to the development of public policies for cancer.
Funding
The American Cancer Society, the National Cancer Institute of the National Institutes of Health, the Centers for Disease Control and Prevention, the Central Brain Tumor Registry of the United States, and the North American Association of Central Cancer Registries. Funding to pay for the Open Access publication charges associated with the article was provided by the National Cancer Institute.
Supplementary Material
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors would like to acknowledge the contribution of central population- and hospital-based cancer registry staff who collected and compiled the incidence data used in this study. In addition, we thank Andrew Lake, Martin Krapcho, and Rick Firth, of Information Management Services, Inc, for assisting in statistical analyses; Dr Janet Bruner, who reviewed the brain site/histology combinations; Dr Susan Chang, for her input on our early draft; and the Central Brain Tumor Registry of the United States for supporting Dr Bridget McCarthy’s efforts and expertise.
References
- 1.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality, 1973-1995: a report card for the U.S. Cancer. 1998;82(6):1197–1207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91(8):675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 3.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88(10):2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93(11):824–842. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 6.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 8.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 9.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107(8):1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 10.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritz A, Percy C, Jack A. International Classification of Diseases of Oncology. 3rd ed. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 14.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2007. Bethesda, MD: National Cancer Institute; 2010. http://seer.cancer.gov/csr/1975_2007. Accessed September 29, 2010. [Google Scholar]
- 15.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 9 Regs and SEER 13 Regs Research Data, Nov 2009 Sub (1973–2007) and population estimate. http://seer.cancer.gov/popdata/index.html, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission. http://seer.cancer.gov/resources. Accessed September 20, 2010. [Google Scholar]
- 16.Central Brain Tumor Registry of the United States (CBTRUS).; CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2010. [Google Scholar]
- 17.Louis DN, Ohgaki H, Wiestler OD, Cavanee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer. 3rd ed. Cancer. 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–136. [PubMed] [Google Scholar]
- 20.World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th rev. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 21.World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Adapted for Use in the United States. 6th rev. Geneva, Switzerland: World Health Organization; 1948. [Google Scholar]
- 22.World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Adapted for Use in the United States. 7th rev. Geneva, Switzerland: World Health Organization; 1955. [Google Scholar]
- 23.US Department of Health, Education, and Welfare. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Adapted for Use in the United States. 8th rev. Washington, DC: US Department of Health, Education, and Welfare, National Center for Health Statistics, Public Health Service; 1968. [Google Scholar]
- 24.World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, Based on the Recommendations of the Ninth Revision Conference, 1975. Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 25.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008;(148):1–23. [PubMed] [Google Scholar]
- 26.National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) Program. Population Estimates Used in NCI’s SEER*Stat Software. http://seer.cancer.gov/popdata/methods.html. Accessed March 8, 2011. [Google Scholar]
- 27.Centers for Disease Control and Prevention. National Center for Health Statistics. National Vital Statistics System. U.S. Census Populations With Bridged Race Categories. http://www.cdc.gov/nchs/nvss/bridged_race.htm. Accessed March 8, 2011. [Google Scholar]
- 28.Surveillance Research Program. National Cancer Institute SEER*Stat Software, version 6.6.2. Bethesda, MD: National Cancer Institute; 2010. http://www.seer.cancer.gov/seerstat. Accessed September 20, 2010. [Google Scholar]
- 29.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 31.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900–80. J Natl Cancer Inst. 1983;71(3):473–479. [PubMed] [Google Scholar]
- 33.Burns DM, Lee L, Shen LZ, et al. Cigarette Smoking Behavior in the United States. Changes in Cigarette-Related Disease Risks and Their Implication for Prevention and ControlBethesda, MD. National Institutes of Health, National Cancer Institute; Monograph 8. NIH Pub No. 97–4213; 1997. [Google Scholar]
- 34.Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93(4):277–283. doi: 10.1093/jnci/93.4.277. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, Ward E, Thun MJ. Contemporary lung cancer trends among U.S. women. Cancer Epidemiol Biomarkers Prev. 2005;14(3):582–585. doi: 10.1158/1055-9965.EPI-04-0554. [DOI] [PubMed] [Google Scholar]
- 36.Saraiya M, Glanz K, Briss PA, et al. Interventions to prevent skin cancer by reducing exposure to ultraviolet radiation: a systematic review. Am J Prev Med. 2004;27(5):422–466. doi: 10.1016/j.amepre.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Bishop JN, Bataille V, Gavin A, et al. The prevention, diagnosis, referral and management of melanoma of the skin: concise guidelines. Clin Med. 2007;7(3):283–290. doi: 10.7861/clinmedicine.7-3-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 39.Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 42.Tsodikov A, Szabo A, Wegelin J. A population model of prostate cancer incidence. Stat Med. 2006;25(16):2846–2866. doi: 10.1002/sim.2257. [DOI] [PubMed] [Google Scholar]
- 43.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19(2):175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holford TR, Cronin KA, Mariotto AB, Feuer EJ. Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006;36:19–25. doi: 10.1093/jncimonographs/lgj016. [DOI] [PubMed] [Google Scholar]
- 46.DeSantis C, Howlader N, Cronin KA, Jemal A, et al. Breast cancer incidence rates in US women are no longer declining [published online ahead of print Feb 28, 2011] Cancer Epidemiol Biomarkers Prev. 2011. doi: 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 47.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356(16):1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 48.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9(3):R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 50.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerlikowske K. Evidence-based breast cancer prevention: the importance of individual risk. Ann Intern Med. 2009;151(10):750–752. doi: 10.7326/0003-4819-151-10-200911170-00012. [DOI] [PubMed] [Google Scholar]
- 52.Mandelblatt JS, Cronin KA, Bailey S. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saslow D, Boetes C, Burke W, et al. American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. Erratum in: CA Cancer J Clin. 2007;57(3):185. [DOI] [PubMed] [Google Scholar]
- 54.Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society High-Risk Work Group, American Cancer Society Screening Older Women Work Group, American Cancer Society Mammography Work Group, American Cancer Society Physical Examination Work Group, American Cancer Society New Technologies Work Group, American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53(3):141–169. doi: 10.3322/canjclin.53.3.141. [DOI] [PubMed] [Google Scholar]
- 55.Garber JE. Breast cancer screening: a final analysis? CA Cancer J Clin. 2003;53(3):138–140. doi: 10.3322/canjclin.53.3.138. [DOI] [PubMed] [Google Scholar]
- 56.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 58.Sloane D. Cancer epidemiology in the United States: racial, social, and economic factors. Methods Mol Biol. 2009;(471):65–83. doi: 10.1007/978-1-59745-416-2_4. [DOI] [PubMed] [Google Scholar]
- 59.Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91(16):1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 60.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 61.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 62.McCarthy BJ, Propp JM, Davis FG, Burger PC. Time trends in oligodendroglial and astrocytic tumor incidence. Neuroepidemiology. 2008;30(1):34–44. doi: 10.1159/000115440. [DOI] [PubMed] [Google Scholar]
- 63.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 64.Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002;95(1):193–202. doi: 10.1002/cncr.10643. [DOI] [PubMed] [Google Scholar]
- 65.Prados MD, et al. Primary neoplasms of the central nervous system in adults. In: Kufe DW, Bast RC Jr, Hait W, editors. Cancer Medicine. 7th ed. Hamilton, ON: BC Decker; 2006. [Google Scholar]
- 66.Kirsch DG, Tarbell NJ. Conformal radiation therapy for childhood CNS tumors. Oncologist. 2004;9(4):442–450. doi: 10.1634/theoncologist.9-4-442. [DOI] [PubMed] [Google Scholar]
- 67.Packer RJ. Childhood brain tumors: accomplishments and ongoing challenges. J Child Neurol. 2008;23(10):1122–1127. doi: 10.1177/0883073808320758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 69.Merchant TE, Pollack IF, Loeffler JS. Brain tumors across the age spectrum: biology, therapy, and late effects. Semin Radiat Oncol. 2010;20(1):58–66. doi: 10.1016/j.semradonc.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connelly JM, Malkin MG. Environmental risk factors for brain tumors. Curr Neurol Neurosci Rep. 2007;7(3):208–214. doi: 10.1007/s11910-007-0032-4. [DOI] [PubMed] [Google Scholar]
- 71.Davis FS. Epidemiology of brain tumors. Expert Rev Anticancer Ther. 2007;7(12 suppl):S3–S6. doi: 10.1586/14737140.7.12s.S3. [DOI] [PubMed] [Google Scholar]
- 72.Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007;25(4):867–890. doi: 10.1016/j.ncl.2007.07.002. vii. [DOI] [PubMed] [Google Scholar]
- 73.Bondy ML, Scheurer ME, Malmer B, et al. Brain Tumor Epidemiology Consortium. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113(7 suppl):1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 75.Gu J, Liu Y, Kyritsis AP, Bondy ML. Molecular epidemiology of primary brain tumors. Neurotherapeutics. 2009;6(3):427–435. doi: 10.1016/j.nurt.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Shete S, Hosking F, Robertson L, Houlston R, Bondy M. Genetic advances in glioma: susceptibility genes and networks. Curr Opin Genet Dev. 2010;20(3):239–244. doi: 10.1016/j.gde.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Shete S, Hosking FJ, Robertson LB, Bondy ML, Houlston RS. New insights into susceptibility to glioma. Arch Neurol. 2010;67(3):275–278. doi: 10.1001/archneurol.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hill DA, Inskip PD, Shapiro WR, et al. Cancer in first-degree relatives and risk of gliomas in adults. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1443–1448. [PubMed] [Google Scholar]
- 80.Hemminki K, Li X. Familial risks in nervous system tumors. Cancer Epidemiol Biomarkers Prev. 2003;12(11, pt 1):1137–1142. [PubMed] [Google Scholar]
- 81.Wrensch M, Lee M, Miike R, et al. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997;145(7):581–593. doi: 10.1093/oxfordjournals.aje.a009154. [DOI] [PubMed] [Google Scholar]
- 82.Hemminki K, Tretli S, Olsen JH, et al. Familial risks in nervous system tumours: joint Nordic study. Br J Cancer. 2010;102(12):1786–1790. doi: 10.1038/sj.bjc.6605708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiemels JL, Wilson D, Patil C, et al. IgE, allergy, and risk of glioma: update from the San Francisco Bay Area Adult Glioma Study in the temozolomide era. Int J Cancer. 2009;125(3):680–687. doi: 10.1002/ijc.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Il’yasova D, McCarthy B, Marcello J, et al. Association between glioma and history of allergies, asthma, and eczema: a case-control study with three groups of controls. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1232–1238. doi: 10.1158/1055-9965.EPI-08-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brenner AV, Linet MS, Fine HA, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99(2):252–259. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 86.Ryan P, Lee MW, North B, McMichael AJ. Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. Int J Cancer. 1992;51(1):20–27. doi: 10.1002/ijc.2910510105. [DOI] [PubMed] [Google Scholar]
- 87.Cicuttini FM, Hurley SF, Forbes A, et al. Association of adult glioma with medical conditions, family and reproductive history. Int J Cancer. 1997;71(2):203–207. doi: 10.1002/(sici)1097-0215(19970410)71:2<203::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 88.Hochberg F, Toniolo P, Cole P, Salcman M. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol. 1990;8(1):55–60. doi: 10.1007/BF00182087. [DOI] [PubMed] [Google Scholar]
- 89.Schlehofer B, Blettner M, Becker N, Martinsohn C, Wahrendorf J. Medical risk factors and the development of brain tumors. Cancer. 1992;69(10):2541–2547. doi: 10.1002/1097-0142(19920515)69:10<2541::aid-cncr2820691025>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 90.Schoemaker MJ, Swerdlow AJ, Hepworth SJ, McKinney PA, van Tongeren M, Muir KR. History of allergies and risk of glioma in adults. Int J Cancer. 2006;119(9):2165–2172. doi: 10.1002/ijc.22091. [DOI] [PubMed] [Google Scholar]
- 91.Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98(4):609–615. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 92.Wigertz A, Lonn S, Schwartzbaum J, et al. Allergic conditions and brain tumor risk. Am J Epidemiol. 2007;166(8):941–950. doi: 10.1093/aje/kwm203. [DOI] [PubMed] [Google Scholar]
- 93.Schlehofer B, Blettner M, Preston-Martin S, et al. Role of medical history in brain tumour development. Results from the International Adult Brain Tumour Study. Int J Cancer. 1999;82(2):155–160. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 94.Schwartzbaum J, Jonsson F, Ahlbom A, et al. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. Int J Cancer. 2003;106(3):423–428. doi: 10.1002/ijc.11230. [DOI] [PubMed] [Google Scholar]
- 95.Turner MC, Chen Y, Krewski D, Ghadirian P, Thun MJ, Calle EE. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005;162(3):212–221. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 96.Berg-Beckhoff G, Schüz J, Blettner M, et al. History of allergic disease and epilepsy and risk of glioma and meningioma (INTERPHONE study group, Germany) Eur J Epidemiol. 2009;24(8):433–440. doi: 10.1007/s10654-009-9355-6. [DOI] [PubMed] [Google Scholar]
- 97.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99(20):1544–1550. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 98.Schoemaker MJ, Swerdlow AJ, Hepworth SJ, van Tongeren M, Muir KR, McKinney PA. History of allergic disease and risk of meningioma. Am J Epidemiol. 2007;165(5):477–485. doi: 10.1093/aje/kwk048. [DOI] [PubMed] [Google Scholar]
- 99.Ahlbom A, Feychting M, Green A, Kheifets L, Savitz DA, Swerdlow AJ ICNIRP (International Commission for Non-Ionizing Radiation Protection) Standing Committee on Epidemiology. Epidemiologic Evidence on Mobile Phones and Tumor Risk: A Review. Stockholm, Sweden: Department of Epidemiology, Institute of Environmental Medicine, Karolinska Institutet; 2009. [DOI] [PubMed] [Google Scholar]
- 100.Kundi M. The controversy about a possible relationship between mobile phone use and cancer. Environ Health Perspect. 2009;117(3):316–324. doi: 10.1289/ehp.11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khurana VG, Teo C, Kundi M, Hardell L, Carlberg M. Cell phones and brain tumors: a review including the long-term epidemiologic data. Surg Neurol. 2009;72(3):205–214. doi: 10.1016/j.surneu.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 102.Han YY, Kano H, Davis DL, Niranjan A, Lunsford LD. Cell phone use and acoustic neuroma: the need for standardized questionnaires and access to industry data. Surg Neurol. 2009;72(3):216–222. doi: 10.1016/j.surneu.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 103.Inskip PD, Hoover RN, Devesa SS. Brain cancer incidence trends in relation to cellular telephone use in the United States. NeuroOncol. 2010;12(11):1147–1151. doi: 10.1093/neuonc/noq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Howlader N, Ries LA, Stinchcomb DG, Edwards BK. The impact of underreported Veterans Affairs data on national cancer statistics: analysis using population-based SEER registries. J Natl Cancer Inst. 2009;101(7):533–536. doi: 10.1093/jnci/djn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19(3):227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howe HL, Lake A, Schymura MJ, Edwards BK. Indirect method to estimate specific Hispanic group cancer rates. Cancer Causes Control. 2009;20(7):1215–1226. doi: 10.1007/s10552-009-9398-8. [DOI] [PubMed] [Google Scholar]
- 107.Espey DK, Wiggins C, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native Populations. Cancer. 2008;113(S5):1120–1130. doi: 10.1002/cncr.23724. [DOI] [PubMed] [Google Scholar]
- 108.Wingo PA, Tucker TC, Jamison PM, et al. Cancer in Appalachia, 2001-2003. Cancer. 2008;112(1):181–192. doi: 10.1002/cncr.23132. [DOI] [PubMed] [Google Scholar]
- 109.Lengerich EJ, Tucker TC, Powell RK, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: disparities in Appalachia. J Rural Health. 2005;21(1):39–47. doi: 10.1111/j.1748-0361.2005.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 110.Becker TM, Espey DK, Lawson HW, Saraiya M, Jim MA, Waxman AG. Regional differences in cervical cancer incidence among American Indians and Alaska Natives, 1999-2004. Cancer. 2008;113(5 suppl):1234–1243. doi: 10.1002/cncr.23736. [DOI] [PubMed] [Google Scholar]
- 111.Rajaraman P, Brenner AV, Butler MA, et al. Common variation in genes related to innate immunity and risk of adult glioma. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1651–1658. doi: 10.1158/1055-9965.EPI-08-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKean-Cowdin R, Barnholtz-Sloan J, Inskip PD, et al. Associations between polymorphisms in DNA repair genes and glioblastoma. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1118–1126. doi: 10.1158/1055-9965.EPI-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kiuru A, Lindholm C, Heinävaara S, et al. XRCC1 and XRCC3 variants and risk of glioma and meningioma. J Neurooncol. 2008;88(2):135–142. doi: 10.1007/s11060-008-9556-y. [DOI] [PubMed] [Google Scholar]
- 114.Malmer B, Adatto P, Armstrong G, et al. GLIOGENE an International Consortium to Understand Familial Glioma. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1730–1734. doi: 10.1158/1055-9965.EPI-07-0081. [DOI] [PubMed] [Google Scholar]
- 115.Moore HM, Compton CC, Lim MD, Vaught J, Christiansen KN, Alper J. 2009 Biospecimen research network symposium: advancing cancer research through biospecimen science. Cancer Res. 2009;69(17):6770–6772. doi: 10.1158/0008-5472.CAN-09-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khleif SN, Doroshow JH, Hait WN AACR-FDA-NCI Cancer Biomarkers Collaborative. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin Cancer Res. 2010;16(13):3299–3318. doi: 10.1158/1078-0432.CCR-10-0880. [DOI] [PubMed] [Google Scholar]
- 117.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.US Census Bureau. US Population Projections. Table 5: Population under age 18 and 65 and older: 2000, 2010, and 2030. US Census Bureau/US Population Projections Web site. http://www.census.gov/population/www/projections/projectionsagesex.html. Accessed March 8, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.