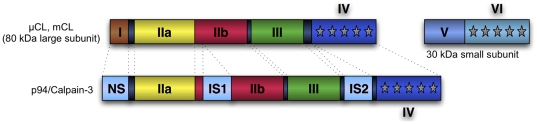
Figure 1. Schematic structures of major calpain homologues.
“Conventional” calpains (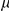 - and m-calpain) are composed of larger catalytic subunits (calpain-1 and -2) and a smaller regulatory subunit. Some homologues, such as skeletal muscle-specific calpain (calpain-3/p94) have slightly diverged properties, including unique insertion sequences (NS, IS1 and IS2) and no requirement for a small subunit. Symbols used are: I: N-terminal domain with little homology; IIa and IIb: protease sub-domains containing the active sites Cys and His/Asn, respectively; III: C2-like
- and m-calpain) are composed of larger catalytic subunits (calpain-1 and -2) and a smaller regulatory subunit. Some homologues, such as skeletal muscle-specific calpain (calpain-3/p94) have slightly diverged properties, including unique insertion sequences (NS, IS1 and IS2) and no requirement for a small subunit. Symbols used are: I: N-terminal domain with little homology; IIa and IIb: protease sub-domains containing the active sites Cys and His/Asn, respectively; III: C2-like 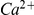 -binding domain; IV and VI: 5-EF-hand
-binding domain; IV and VI: 5-EF-hand 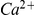 -binding domain; V: Gly-rich hydrophobic domain; NS, IS1 and IS2: p94-specific sequences.
-binding domain; V: Gly-rich hydrophobic domain; NS, IS1 and IS2: p94-specific sequences.