Figure 3.
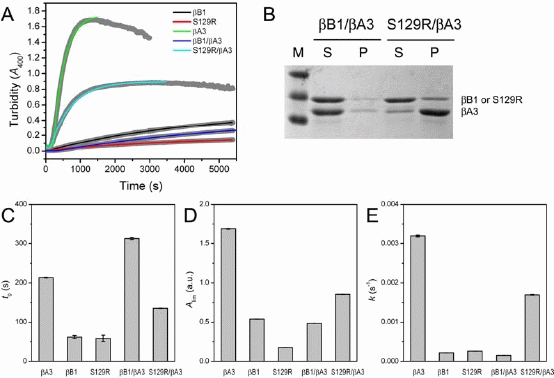
Effect of the p.Ser129Arg mutation on the thermal stability of βB1-crystallin homomer and βB1/βA3crystallin heteromer. (A) The time-course aggregation kinetics monitored by the turbidity at 400 nm at 60°C. The raw data was fitted by Eq. 1, and the kinetic parameters were presented in panels (C-E). The decrease of the turbidity of βA3-crystallin and S129R/βA3-crystallin after long time incubation was caused by the deposition of the large aggregates, and was not included in the curve fitting. (B) SDS-PAGE analysis of the aggregates separated by centrifugation. S and P represent supernatant and precipitate, respectively.
