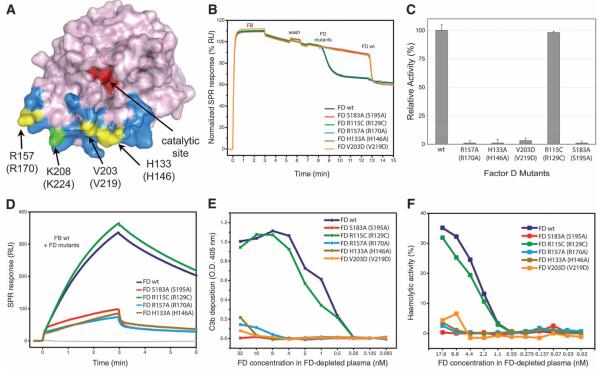
Fig. 3.
Analysis of FD exosite. (A) Surface diagram of FD highlighting its exosite in blue and its catalytic site in red (28). Exosite mutations are shown in yellow, whereas the biotinylation site associated with FD inactivation (26) is shown in green. (B) FD-mediated cleavage of C3bB pro-convertases monitored by SPR; FD mutants were injected onto surface-bound C3bB, and the cleavage activity was compared with that of wild type (wt). A drop in SPR response upon FD injection corresponds to the removal of the Ba fragment. Wild-type FD was injected at the end of each experiment to ensure cleavage sensitivity. (C) FD-mediated cleavage of C3bB performed by using SDS-PAGE with FD exosite mutants. The histogram shows the relative pro-convertase activation rates compared with those of wild-type FD. Error bars represent deviations from the mean observed in multiple experiments (n>3). (D) Effect of FD exosite mutations on the formation and decay of the C3bBb convertase monitored by SPR; wild-type FB was premixed with various FD mutants and injected over immobilized C3b. RU, resonance units. (E) Reconstitution of complement activity in FD-depleted plasma using FD mutants determined as C3b deposition on the enzyme-linked immunosorbent assay (ELISA) plate. O.D., optical density. (F) Hemolytic activity assays using FD-depleted plasma reconstituted with different mutants of FD: Lysis of rabbit erythrocytes was monitored by colorimetry and compared with 100% haemolysis in water.