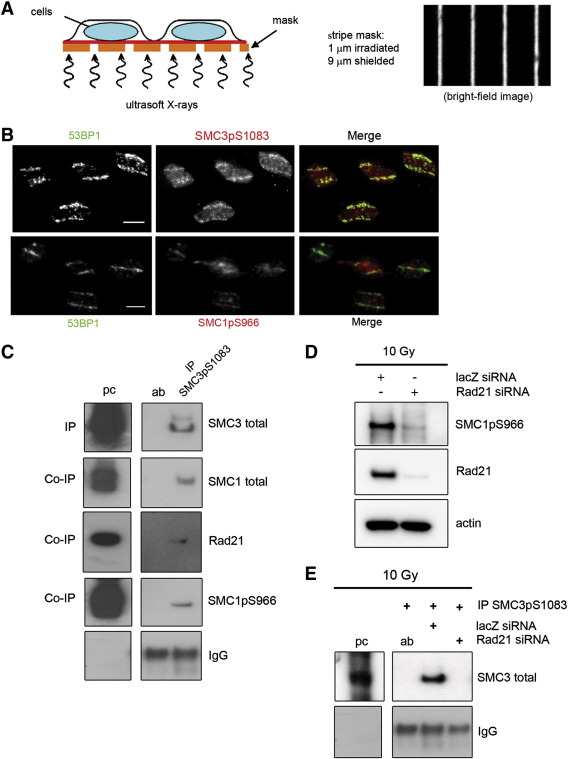
Fig. 1.
SMC1 and SMC3 are phosphorylated when part of the cohesin complex. (A) Defined ‘stripe’ patterns of localized DNA damage within the nucleus were achieved by using ultrasoft X-rays and a grid which contains 9 μm wide gold plates separated by a 1 μm wide open area as seen in the bright field microscopy image. (B) HeLa cells were grown on 0.9 μm thick Mylar for 24 h and then irradiated through the grid and the Mylar with ultrasoft X-rays. Cells were left for 1 h before fixation to allow DNA repair. Immunofluorescence microscopy for 53BP1 to detect areas of DNA damage and for SMC3pS1083 or SMC1pS966 was performed 1 h after ultrasoft X-ray irradiation. Scale bar 10 μm. (C–E) One representative blot is shown, two independent repeats. (C) 100 μg of un-irradiated or 10 Gy X-irradiated HeLa cell extracts was used for immuno-precipitation (IP) of SMC3pS1083. Precipitated proteins were analyzed by Western blotting. Panel shows (from top to bottom) precipitated SMC3 protein, co-precipitated total SMC1, Rad21 and SMC1pS966 and precipitated IgG antibody band. For lane ‘pc’ (positive control) 10 μg of X-irradiated extract (10 Gy) was loaded. Lane ‘ab’ shows the precipitated antibody. (D) HeLa cells were either transfected with non-specific siRNA (lacZ) or siRNA targeting Rad21. 20 μg of extract was loaded in each lane and Rad21 and SMC1pS966 levels were analyzed. Actin was used as loading control. (E) 100 μg of HeLa extracts treated with siRNAs for non-specific control (lacZ) or Rad21 was used for immuno-precipitation of SMC3pS1083. The precipitated SMC3 levels were analyzed on a Western blot. Antibody bands were used as loading control. For lane ‘pc’ 20 μg of X-irradiated extract (10 Gy) was loaded.