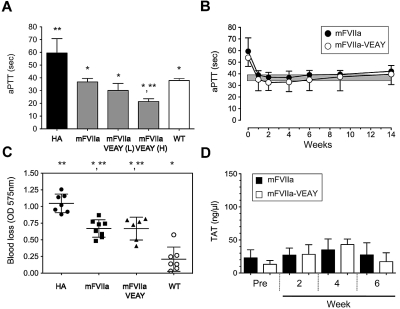
Figure 3.
Hemostatic efficacy of mFVIIa-VEAY after gene transfer in hemophilia A mice. (A) Dose escalation study in HA mice receiving 1.2 × 1012 vg of AAV-mFVIIa, 1.2-3 × 1010 vg of AAV-mFVIIa-VEAY (low dose, mFVIIa-VEAY [L]) or 0.6-0.3 × 1012 vg of AAV-mFVIIa-VEAY (high dose, mFVIIa-VEAY [H]). Activated partial thromboplastin time (aPTT) values are shown for samples assayed at 6 weeks post AAV administration. Wild-type (WT) and HA aPTTs are also shown. Each cohort consisted of at least 3 mice. *P < .009 vs HA; **P < .007 vs WT. All AAV vectors were of serotype 8. (B) aPTT in HA mice after delivery of AAV-mFVIIa (1.2 × 1012 vg/mouse) or AAV-mFVIIa-VEAY (1.2-3 × 1010 vg/mouse). Each cohort consisted of 10 mice. The gray box indicates the range of aPTT values using human FVIII deficient plasma in hemostatically normal mice (37.9 ± 1.6 seconds, n = 10). (C) Blood loss after a tail clip injury in AAV-treated HA mice compared with wild-type (WT) and HA controls. *P < .002 compared with HA; **P < .001 vs WT. (D) TAT levels compared with baseline (Pre) in HA mice after gene transfer. All data are shown as average ± 1 SD.