Abstract
Background
Coffee consumption has been shown to be inversely associated to type 2 diabetes mellitus (T2DM), but evidence in Chinese populations is limited. We investigated the relationship between coffee consumption and T2DM in a population-based cohort of middle-aged Chinese.
Materials and Methods
We studied 2,332 subjects who participated in the Taichung Community Health Study in Taiwan in 2004. The relationships between coffee consumption, T2DM and fasting glucose were assessed.
Results
The prevalence of T2DM was 14.0% and 10.4% in men and women. After adjustment for age, body mass index, blood pressure, smoking, alcohol drinking, betel nut chewing, physical activity, income, education level, fat%, protein%, carbohydrate%, and magnesium, coffee intake was inversely associated with T2DM. Habitual coffee drinkers had 38–46% lower risk of T2DM than non-drinkers. Compared to non-drinkers, the adjusted odds ratios (ORs) for T2DM according to subjects with habitual coffee consumption (<1, 1–6, ≥ 7 times per week) were 0.77(0.52–1.13), 0.46(0.28–0.76), and 0.37(0.16–0.83), respectively. The decreasing ORs indicate a dose-response effect of coffee consumption on the likelihood of having T2DM (p < 0.001). A similar relationship was also evident in newly-diagnosed T2DM (p < 0.05). The adjusted mean fasting glucose levels gradually decreased as the frequency of coffee consumption increased (p < 0.05).
Conclusions
Coffee intake is inversely associated with T2DM in Chinese. Coffee may be a protective agent for T2DM in Chinese.
Keywords: coffee, type 2 diabetes, Chinese, dose-response, glucose
Introduction
Type 2 diabetes mellitus (T2DM) is one of the leading causes of death in the world [1, 2]. The number of type 2 diabetic patients has dramatically increased, especially in the developing countries. It has been estimated that 60 million new cases of T2DM will occur worldwide from 2000 to 2010 [3]. The International Diabetes Federation estimated that the diabetes population will reach 380 million globally by 2025 [2]. Perhaps as a result of increasing westernized diet habits and physical inactivity, the prevalence of obesity as well as T2DM in Taiwan had increased in past decades. The prevalence of T2DM in middle-aged adults increased steadily from 5.1% to 8.2% to 12.8% in 1970, 1986, and 1993, respectively [4, 5]. By 1999, the prevalence of diabetes reached 13.0% in men and 16.1% in women for those aged above 53 [6]. Among men aged 65 years and above, in National Nutrition Survey in Taiwan, it increased dramatically from 13.1% to 17.6% to 28.5% in 1993–1996, 2002, and 2005–2008, respectively [7]. The International Diabetes Federation proposed that the causes of increase of diabetes prevalence were because of population aging, unhealthy diet, obesity, and a sedentary lifestyle [2]. Evidences also found that psychosocial stress, depression, and environmental pollutants may affect the risk for T2DM [8–10]. The prevalence of T2DM in Taiwan is now as high as in the United States [1], despite a much lower prevalence of overweight and obesity. In China, the prevalence of diabetes were 9.7% (10.6% among men and 8.8% among women) whcih accounted for 92.4 million adults with diabetes and it increased with increasing age [11]. Alongside aging and obesity, unhealthy diet and a sedentary lifestyle are major determinants for the risk of T2DM. Adult leisure-time physical activity appears to increasing worldwide [12], but dietary habits may vary between countries. For example, although coffee consumption has increased four-fold from 1993 to 2003 in Taiwan [13], the prevalence is still far less than the United States, where more than half of adults drink coffee [14]. Huxley et al [15] reported that an inverse relationship between coffee consumption and subsequent risk of diabetes in a systematic review with meta-analysis in Caucasian. However, data for Asian populations (which are characterised by considerably increasing numbers of diabetes patients) are scarce [16]. For example, in the Singapore Chinese Health Study, Odegaard et al [16] reported that regular coffee consumption was associated with lower risk of T2DM in Singapore Chinese. Similar studies were also found in Europe, Japan, and the United States [17–19]. However, Saremi and colleagues found that there was no significant association between coffee consumption and incidence of diabetes among Pima Indians[20], indicating a possible racial disparity. China has recently overtaken India as the global epicenter of the diabetes epidemic, with over 92.4 million adults reportedly having the disease [21]. Thus, identifying potential dietary risk or protective factors for T2DM has become an urgent research topic in China. Although Odegaard et al [16] recently reported an inverse association between coffee consumption and diabetes in Singapore Chinese, dietary habits may be quite different between Singapore and China. The Taiwanese population more closely represents China with regards to race and lifestyle factors and is better suited to evaluate the effects of lifestyle on T2DM prevalence. Our aim was to assess the association between coffee intake and T2DM after adjusting for age, obesity, lifestyle factors, dietary factors, and other potential confounders in a Chinese population of middle-aged adults in Taiwan.
Materials and Methods
Study population
The target population consisted of residents aged 40 and above in Taichung city (an urban city), Taiwan, in October, 2004. There were a total of 363,543 residents in this area during the time of study, which represented about 4.09% of the national population of the same age. The detailed sampling method has been described in previous reports [22–24]. In brief, a two-stage sampling design was used to identify residents, with a sampling rate proportional to size within each stage. Out of 363,543 residents, 2359 subjects were recruited. These subjects represent an urban Taiwanese population. Subjects with incomplete data for coffee consumption were excluded, so that the final population was 2332 subjects. The selected (n=2332) and non-selected (n=27) groups did not differ by age, gender, BMI, waist circumference, or serum glucose.
Anthropometric indices and laboratory assays
Height, weight, waist circumference, and BP were measured by trained staff. Body mass index was calculated as weight (kg) divided by height squared (m2). Blood was drawn in the morning after a 12-hour overnight fast and was sent for analysis within 4h of collection. Total cholesterol, HDL-C, triglycerides, and fasting glucose were analyzed with a biochemical autoanalyzer (Beckman Cou, Fullerton, CA, USA) at the Clinical Laboratory Department (China Medical University Hospital, Taichung, Taiwan).
Sociodemographic factors and life style behaviors
Age, gender, employment, education, physical activity and medical history were collected by self-administered questionnaires. Smoking, alcohol drinking, and betel nut chewing history were divided into 3 classes as follows: never, former, and current. Physical activity status was divided into 2 classes: never/seldom and current. Diet habits were collected by food frequency questionnaires. The standard serving size for coffee consumption was assigned on the questionnaire as 1 cup (240 ml). The frequency of coffee intake ranged from “never” up to “7 times or more per week”. Average coffee consumption was defined as the average frequency of habitual coffee intake in the past 6 months, recorded as 0, <1, 1–6, ≥7 times per week. Non-drinkers were defined as 0 times per week, the others were defined as habitual coffee drinkers. Decaffeinated coffee is rarely consumed in Taiwan, so we assessed coffee consumption only. Most coffee drinkers added sugar and milk (or cream) into their coffee, so separate analyses were not conducted to assess the influence of coffee additives. Tea consumption was defined as the average frequency of habitual tea intake in past 6 months and analyzed as habitual drinkers vs. non-drinkers, as was done with coffee consumption. Income was divided into 3 levels: low (< USD 15,000/year), middle (USD 15,000–37,500/year), and high (>USD 37,500/year). Education was also divided into three levels: low (elementary school and below), middle (junior and senior high school), and high (college/university and above).
Definition of type 2 diabetes
Type 2 diabetes was defined as a fasting plasma glucose concentration ≥ 7.0 mmol/l (126 mg/dl) and/or history of T2DM and on hypoglycemic agent treatment or insulin treatment. New diagnosed T2DM was defined as a fasting plasma glucose concentration ≥ 7.0 mmol/l without history of T2DM.
Statistical analysis
The data are presented as means and SD unless otherwise indicated. Student’s t-test was used to compare mean values. Log transformation was used for variables (age, height, weight, BMI, waist circumference, systolic BP, diastolic BP, fasting glucose, triglycerides, HDL-C, total cholesterol/HDL-C) with significant deviation from normal distribution, and assessed by the Kolmogorov–Smirnov test before further analyses. Pearson’s χ2 test was used to compare the differences in the categorical variables (such as smoking and alcohol drinking) according to diabetic status. ANOVA test was used to compare the continuous variables across coffee consumption status. Multiple logistic regression analyses were used to assess the association between T2DM and coffee consumption status. All statistical tests were 2-sided at the 0.05 significance level. These statistical analyses were performed using the PC version of SPSS statistical software (13th version, SPSS Inc., Chicago, IL, USA).
Reporting of the study conforms to STROBE along with references to STROBE and the broader EQUATOR guidelines [25]. Ethics approval for patient recruitment and data analysis was obtained from the Institutional Review Board of the China Medical University Hospital. The informed consent was obtained from every study participant. The reported investigations were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Results
Table 1 shows that subjects with T2DM were older and had greater weight, BMI, WC, systolic BP, diastolic BP, fasting glucose, triglycerides, and total cholesterol/ HDL-cholesterol and lower HDL-cholesterol and magnesium than subjects without T2DM. The prevalence of T2DM was 14.0% in men and 10.4% in women. Table 2 shows the baseline characteristics across the frequency of coffee consumption.
Table 1.
Baseline characteristics of the cohort according to diabetic status
| Diabetes (n=284) | Non-Diabetes(n=2048) | p value | |
|---|---|---|---|
| Male (n, %) | 159 (56%) | 974 (48%) | 0.008 |
| Age (years) | 63.2±11.1 | 56.0±11.4 | <0.001 |
| Height (cm) | 160.9±8.1 | 160.7±8.0 | 0.610 |
| Weight (kg) | 66.0±12.0 | 62.6±10.8 | <0.001 |
| BMI (kg/m2) | 25.4±3.5 | 24.2±3.3 | <0.001 |
| Waist circumference (cm) | 86.2±10.4 | 80.7±9.8 | <0.001 |
| Systolic BP (mmHg) | 147.8±22.1 | 134.0±21.5 | <0.001 |
| Diastolic BP (mmHg) | 83.3±11.9 | 78.4±12.4 | <0.001 |
| Fasting glucose (mmol/l) | 8.86±2.71 | 5.30±0.50 | <0.001 |
| Total cholesterol (mmol/l) | 5.33±1.04 | 5.24±0.97 | 0.153 |
| Triglycerides(mmol/l) | 1.93±1.49 | 1.29±0.97 | <0.001 |
| HDL-C (mmol/l) | 1.07±0.26 | 1.20±0.33 | <0.001 |
| Total cholesterol/HDL-C | 5.17±1.22 | 4.60±1.24 | <0.001 |
| Fat (%) | 17.2±4.8 | 16.8±4.5 | 0.144 |
| Protein (%) | 16.8±3.2 | 16.3±3.1 | 0.008 |
| Carbohydrate (%) | 66.4±7.4 | 68.1±7.6 | <0.001 |
| Magnesium (mg/day) | 269±118 | 295±145 | <0.001 |
| Tea consumption (%) | 65.4 % | 70.0 % | 0.067 |
| Coffee consumption (%) | <0.001 | ||
| 0 time/wk | 76.8% | 59.4% | |
| < 1 time/wk | 13.4% | 19.5% | |
| 1~6 times/wk | 7.4% | 14.6% | |
| ≥7 times/wk | 2.5% | 6.5% | |
| Smoking (%) | 0.002 | ||
| Never | 66.2% | 73.3% | |
| Former | 18.0% | 10.9% | |
| Current | 15.8% | 15.8% | |
| Alcohol drinking | <0.001 | ||
| Never | 70.8% | 72.2% | |
| Former | 10.6% | 4.4% | |
| Current | 18.7% | 23.4% | |
| Betel nut chewing | 0.611 | ||
| Never | 90.1% | 91.0% | |
| Former | 5.3% | 5.6% | |
| Current | 4.6% | 3.4% | |
| Physical activity | 0.771 | ||
| None/seldom | 33.5% | 32.6% | |
| Regular | 66.5% | 67.4% | |
| Income | <0.001 | ||
| Low | 65.3% | 45.6% | |
| Middle | 29.2% | 42.9% | |
| High | 5.5% | 11.5% | |
| Education | <0.001 | ||
| Low | 39.4% | 22.4% | |
| Middle | 37.3% | 41.4% | |
| High | 23.2% | 36.1% |
Student's t-test for unpaired data was used for the comparison of mean values between genders; data are means ± SD;
Pearson’s χ2 test was used for categorical data; data were shown as percentage.
Table 2.
Baseline characteristics categorized by coffee consumption (n=2332)
| 0 time/wk (n=1434) |
<1time/wk (n=437) |
1~6 times/wk (n=321) |
≥7times/wk (n=140) |
p value |
|
|---|---|---|---|---|---|
| Male (n, %) | 691(48.2%) | 212(48.5%) | 160(49.8%) | 70(50.0%) | 0.939 |
| Age (years) | 58.7±12.0 | 54.1±10.0 | 53.8±10.5 | 53.7±10.8 | <0.001 |
| Height (cm) | 160.3±8.0 | 161.0±7.9 | 162.1±8.1 | 160.6±8.2 | 0.003 |
| Weight (kg) | 62.7±10.9 | 63.2±11.3 | 64.4±10.9 | 62.6±10.9 | 0.077 |
| BMI (kg/m2) | 24.3±3.4 | 24.3±3.2 | 24.4±3.3 | 24.2±3.3 | 0.852 |
| Waist circumference (cm) | 81.6±10.2 | 80.8±9.7 | 81.7±9.8 | 80.8±10.5 | 0.416 |
| Systolic BP (mmHg) | 138.2±22.6 | 131.0±20.1 | 133.2±20.9 | 130.9±20.3 | <0.001 |
| Diastolic BP (mmHg) | 79.6±12.5 | 77.8±12.4 | 78.5±12.4 | 77.2±11.9 | 0.010 |
| Glucose (mmol/l) | 5.83±1.69 | 5.64±1.55 | 5.55±1.04 | 5.50±1.28 | 0.002 |
| Total cholesterol (mmol/l) | 5.23±0.98 | 5.23±0.97 | 5.35±0.95 | 5.41±0.97 | 0.042 |
| Triglycerides(mmol/l) | 1.36±0.98 | 1.40±1.25 | 1.40±1.12 | 1.37±1.13 | 0.882 |
| HDL-C (mmol/l) | 1.19±0.33 | 1.18±0.32 | 1.19±0.31 | 1.21±0.35 | 0.729 |
| Total cholesterol/ HDL-C | 4.63±1.21 | 4.69±1.28 | 4.74±1.36 | 4.75±1.37 | 0.436 |
| Fat (%) | 16.7±4.7 | 16.8±4.5 | 17.0±4.0 | 18.0±4.8 | 0.013 |
| Protein (%) | 16.4±3.2 | 16.2±3.0 | 16.2±3.0 | 16.0±2.9 | 0. 240 |
| Carbohydrate (%) | 67.8±7.7 | 68.0±7.6 | 68.2±7.0 | 67.4±7.8 | 0.726 |
| Magnesium (mg/day) | 288±143 | 285±141 | 308±141 | 317±133 | 0.016 |
| Tea consumption (%) | 62.3 % | 81.2 % | 84.3 % | 72.1 % | <0.001 |
| Smoking (%) | <0.001 | ||||
| Never | 72.5% | 75.1% | 73.5% | 61.4% | |
| Former | 12.9% | 10.0% | 9.7% | 8.6% | |
| Current | 14.6% | 14.4% | 16.8% | 30.0% | |
| Alcohol drinking | 0.002 | ||||
| Never | 72.8% | 68.9% | 72.9% | 72.1% | |
| Former | 6.3% | 2.7% | 5.0% | 2.1% | |
| Current | 20.9% | 28.4% | 22.1% | 25.7% | |
| Betel nut chewing | 0.279 | ||||
| Never | 91.0% | 90.6% | 91.8% | 88.6% | |
| Former | 5.9% | 5.9% | 3.1% | 6.4% | |
| Current | 3.1% | 3.4% | 5.0% | 5.0% | |
| Physical activity | 0.001 | ||||
| None/seldom | 30.1% | 37.3% | 33.3% | 43.6% | |
| Regular | 69.9% | 62.7% | 66.7% | 56.4% | |
| Income | <0.001 | ||||
| Low | 52.8% | 38.4% | 40.8% | 44.5% | |
| Middle | 37.8% | 47.2% | 46.5% | 46.0% | |
| High | 9.3% | 14.4% | 12.7% | 9.5% | |
| Education | <0.001 | ||||
| Low | 29.9% | 14.9% | 14.7% | 22.1% | |
| Middle | 39.0% | 44.5% | 42.8% | 45.0% | |
| High | 31.1% | 40.6% | 42.5% | 32.9% |
ANOVA test was used for comparing mean values of continuous variables between groups; data were shown as means ± SD;
Pearson’s χ2 test was used for categorical data; data were shown as percentage.
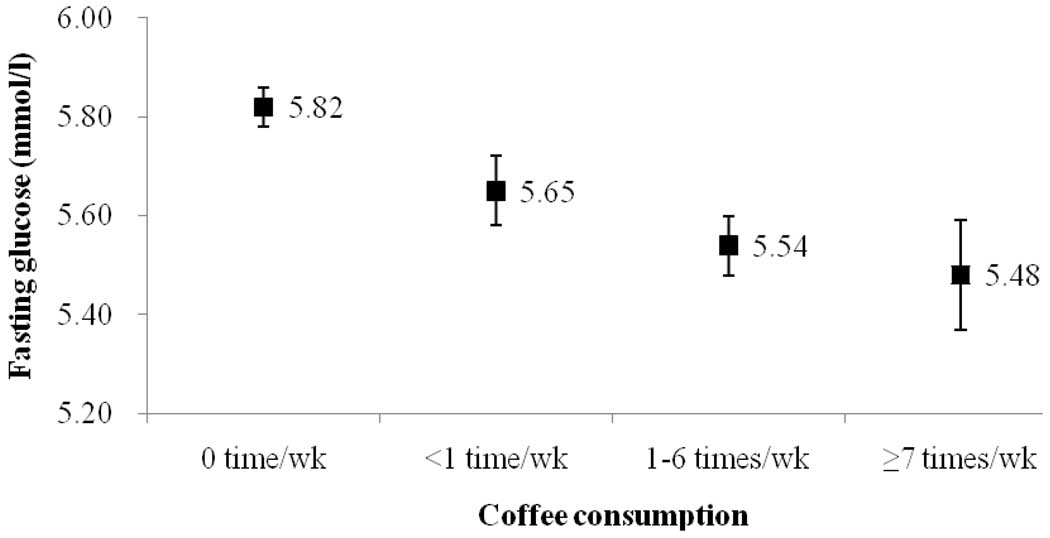
After adjusting for the effects of age, BMI, systolic BP, diastolic BP, smoking, alcohol drinking, betel nut chewing, physical activity, income, education level, fat, protein, carbohydrate, and magnesium in multiple logistic regression analyses, the adjusted OR for T2DM with habitual coffee drinkers was lower than non-drinkers (Table 3). The adjusted OR (95% CI) was 0.62(0.41–0.92) in men and 0.54(0.31–0.92) in women (Table 3). To further clarify the dose response effect, we analyzed the association between T2DM and frequency of coffee consumption in Table 4. Using multiple logistic regression analyses with adjustment for potential confounders, the adjusted OR for T2DM were significantly lower among drinkers with higher coffee consumption than among non-drinkers (model 1–4 in Table 4). Compared to non-drinkers, the adjusted OR (95% CI) for T2DM according to subjects with habitual coffee consumption (<1, 1–6, ≥ 7 times per week) was 0.76 (0.52–1.13), 0.45 (0.27–0.74), and 0.36 (0.16–0.82), respectively. The decreasing OR for T2DM with higher coffee consumption demonstrated a dose-response effect (p < 0.001). Furthermore, we assessed the association between newly-diagnosed T2DM and coffee consumption. The prevalence of new-diagnosed T2DM subjects among habitual coffee consumption 0, <1, 1~6, or ≥7 times/wk was 5.9 %, 5.2%, 1.6%, and 2.9%, respectively. Compared to non-drinkers, the adjusted OR for habitual coffee consumption was 0.83(0.44–1.54) in men and 0.46(0.22–0.99) in women. We also found a decreasing OR (0.72(0.55–0.94)) for newly-diagnosed T2DM with higher coffee consumption, also demonstrating a dose-response effect (p = 0.017 for test for trend). Fasting glucose levels were also gradually decreased with increasing frequency of coffee consumption (Figure 1). After adjustment for potential confounders, the adjusted mean fasting glucose levels (±SE) between coffee consumption groups (habitual consumption 0, <1, 1~6, or ≥7 times/wk) were 5.82±0.04, 5.65±0.07, 5.54±0.06, and 5.48±0.11 mmol/l, respectively (p value = 0.002 for test for trend; Figure 1).
Table 3.
Adjusted odds ratios (95% confidence interval) of having diabetes derived from a multiple logistic regression analysis using age, body mass index, and coffee consumption as independent variables in both genders, adjusted for potential confounders.
| Variable | Men (n=1133) | Women (n=1199) |
|---|---|---|
| Age | 1.02(1.00–1.04)a | 1.04(1.01–1.07)b |
| Body mass index | 1.09(1.03–1.15)b | 1.05(0.99–1.11) |
| Coffee consumption (habitual coffee drinkers vs. non-drinkers) | 0.62(0.41–0.92)a | 0.54(0.31–0.92)a |
Adjusted for systolic blood pressure, diastolic blood pressure, smoking, alcohol drinking, betel nut chewing, physical activity, income, education level, fat %, protein %, carbohydrate %, and magnesium
p < 0.05
p < 0.01
Table 4.
Odds ratios (95% confidence interval) of having diabetes in several different models derived from a multiple logistic regression analysis using coffee consumption as independent variables, adjusted for potential confounders
| Variable | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Coffee(0 time/wk) | 1.00(reference) | 1.00(reference) | 1.00(reference) | 1.00(reference) |
| Coffee(< 1 time/wk) | 0.53(0.37–0.76)c | 0.70(0.48–1.03) | 0.77(0.53–1.13) | 0.77(0.52–1.13) |
| Coffee(1–6 times/wk) | 0.39(0.25–0.62)c | 0.48(0.30–0.78)b | 0.49(0.30–0.80)b | 0.46(0.28–0.76)b |
| Coffee(≥ 7times/wk) | 0.29(0.14–0.64)b | 0.37(0.17–0.82)a | 0.36(0.16–0.80)a | 0.37(0.16–0.83)a |
Model 1: unadjusted
Model 2: adjusted for age, gender, BMI, systolic BP, and diastolic BP
Model 3: adjusted for model 2 variables, plus smoking, alcohol drinking, betel nut chewing, physical activity, income, and educational level
Model 4: adjusted for model 3 variables, plus fat %, protein %, carbohydrate %, and magnesium
p < 0.05
p < 0.01
p < 0.001
Figure 1.
Adjusted mean values (±SE) for fasting glucose levels by coffee consumption category (habitual consumption 0 time/wk, <1 time/wk, 1~6 times/wk, or ≥7 times/wk, p value = 0.002 for test for trend), adjustment for age, gender, BMI, systolic BP, diastolic BP, smoking, alcohol drinking, betel nut chewing, physical activity, income, education levels, fat, protein, carbohydrate, and magnesium.
Discussion
These data demonstrate an inverse association between coffee consumption and T2DM prevalence (including newly-diagnosed T2DM) and fasting glucose level independent of many potential confounding anthropometric, lifestyle, and energy intake factors in Chinese middle-aged adults. Coffee consumption also appeared to protect against T2DM in a dose-response manner. These findings are consistent with most studies done in Caucasians [26–28], and carry an important public health message. According to International Diabetes Federation’s estimations, future new cases of T2DM will predominately come from developing countries, especially China. Thus, it is important to investigate strategies that prevent the onset of diabetes in this population. Coffee consumption may be a preventive agent for T2DM in Chinese. Further studies, ideally of prospective design, are warranted to clarify causality in the relationship between coffee intake and the development of T2DM in Chinese.
Several mechanisms have been proposed to explain the association between coffee consumption and T2DM. First, magnesium is a component of coffee, and a higher magnesium intake from food can improve insulin resistance, glycemic control and reduce the risk of T2DM [29, 30]. In our study, we found that serum magnesium levels increased with frequency of coffee intake and subjects without diabetes had greater serum magnesium levels than subjects with diabetes. While magnesium may partially explain the relationship, there was no attenuation of the OR when magnesium entered the model in Table 4. This finding supports the results of Odegaard and colleagues' prospective study in Singapore Chinese [16]. Second, coffee can stimulate thermogenesis and increase energy expenditure, which could result in weight reduction [31]. In fact, some prospective and review studies have reported that coffee consumption's reduction of T2DM risk may be explained by weight reduction [32]. In our study, however, coffee consumption was not associated with BMI, and subjects who consumed more coffee had higher energy intake. Again, when we entered energy intake into the final model, the adjusted OR for T2DM did not weaken. Third, coffee contains anti-oxidants, which promote insulin sensitivity, thus preventing or delaying the development of T2DM [27]. Fourth, the contents of coffee such as chlorogenic acid, quinic acid, trigonelline, and lignin secoisolariciresinol have been reported to improve glucose metabolism [27, 28, 33]. While other mechanisms linking insulin sensitivity and coffee consumption have also been proposed, these others lack definitive evidence. Fifth, coffee consumption may improve subclinical inflammation which represents a potential link between coffee and diabetes risk. Recent intervention trial demonstrated favourable effects of coffee on inflammatory markers in clinical trials [34].
Odegaard's prospective study of Singapore Chinese found that coffee intake of more than 4 cups per day reduced diabetes risk by 30% [16]. In our study, we found that coffee consumption of more than 7 times per week is associated with a 63% lower risk for T2DM. Although our study is cross-sectional in design, the population sample is large enough to reasonably adjust for many potential confounders, including age, BMI, systolic BP, diastolic BP, smoking, alcohol drinking, betel nut chewing, income, education level, energy intake, magnesium, and percentage of fat, protein, and carbohydrate: all of which have been shown to increase the risk of T2DM. Alcohol drinking and carbohydrate rich nutrition were also important lifestyle factors for T2DM. We compared the relative importance of these three lifestyle (coffee consumption, alcohol drinking, and carbohydrate intake) differences between T2DM and Non-T2DM. We put them into Table 3 using multiple logistic regression analyses and we found that only coffee consumption is significantly associated to T2DM among men and women. Therefore, we think that coffee consumption may be the relative important lifestyle factor than others for T2DM in Taiwanese.
There are several limitations to our study. First, the cross-sectional design does not clarify causality. Future prospective cohort studies are necessary to establish causative links. However, the decreasing OR for T2DM and lower fasting glucose level with higher coffee consumption demonstrated dose-response effects, thus supporting the possibility of a causal relation. Second, our study only analyzed the relationship between coffee consumption and T2DM. Previous studies have shown that caffeinated coffee decreases insulin sensitivity and impairs glucose tolerance, which could lead to T2DM. However, some prospective cohort studies have found that decaffeinated coffee consumption is inversely related to incidence of T2DM [17, 27]. Many other ingredients of coffee may play a role in preventing T2DM [29, 30]. Further studies focusing on the relationship between T2DM and components of coffee such as caffeine are necessary. Third, coffee consumption was obtained from a self-report questionnaire, so the potential misclassification of exposure is possible. Similarly, misclassification of T2DM in self-reported medical history was also possible, although we checked each case by hospital medical records. Non-differential misclassification, however, would likely have biased the results to the null, and the significant dose-response effects are evidence of the strength of relationship between coffee consumption and T2DM. Fourth, the information on type of coffee consumed (filtered, unfiltered, instant) was not avaliable. We knew that different types of coffee and different methods of preparation have been described to have different metabolic effects. Therefore, it may confound the study result. Fifth, no oral glucose tolerance tests were performed to diagnose T2DM may cause potential misclassification. Finally, residual confounding is a possible explanation for our finding. For example, the recent study in Singapore Chinese found that black tea, but not green tea reduced the incidence of T2DM [16]. Another study in Japanese found that green tea had an inverse association with T2DM [35]. Tea is another important and commonly consumed beverage in Chinese, especially by the elderly. We therefore adjusted for tea consumption in the model 4 in Table 4, yet found the result to be the same. Thus, the residual confounding effect of tea consumption in our study appears to be minimal.
Coffee is a widespread beverage around the world. There is a rapid increase in coffee consumption in developing countries, including China. Our results report that coffee consumption is inversely related to the prevalence of T2DM and fasting glucose in Chinese. Also, increasing frequency of coffee intake was also inversely associated with the prevalence of T2DM and fasting glucose level, revealing a dose-response effect. With the prevalence of T2DM on the rise and expected to increase dramatically in China, coupled with an apparent growing interest in coffee drinking among the Chinese, the possibility that coffee consumption may provide protection from or in some way mediate this widespread disease has important impact on public health.
Acknowledgements
This study is supported by grants from National Science Council of Taiwan (NSC93-2314-B-039-025, NSC 94-2314-B-039-024), from Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH99-TD-B-111-004), from China Medical University Hospital (DMR-96-118, DMR-97-149, DMR-98-090, and DMR-99-110), and from US National Institutes of Health (DK 026687). We also thank all the staff and subjects who participated in this study.
Footnotes
Disclosure statement
The authors declared no conflict of interest.
Contributor Information
Wen-Yuan Lin, Email: wylin@mail.cmu.edu.tw.
F. Xaiver Pi-Sunyer, Email: fxp1@columbia.edu.
Ching-Chu Chen, Email: chingchu@ms15.hinet.net.
Lance E. Davidson, Email: lance.davidson@utah.edu.
Chiu-Shong Liu, Email: liucs@ms14.hinet.net.
Tsai-Chung Li, Email: tcli@mail.cmu.edu.tw.
Mei-Fong Wu, Email: 68577@cch.org.tw.
Chia-Ing Li, Email: a6446@mail.cmuh.org.tw.
Walter Chen, Email: chenwalt@yahoo.com.
References
- 1.National Institute of Health. National Institute of Diabetes and Digestive and Kidney Disease. National Diabetes Statistics, 2007 fact sheet. [Accessed Nov. 11, 2010];2008 http://diabetes.niddk.nih.gov/DM/PUBS/statistics.
- 2.International Diabetes Federation. [Accessed at Nov. 11, 2010];The Diabetes Atlas, third edition. 2006 http://www.eatlas.idf.org/media/
- 3.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 4.Pan WH, Yeh WT, Chang HY, Hwu CM, Ho LT. Prevalence and awareness of diabetes and mean fasting glucose by age, sex, and region: results from the Nutrition and Health Survey in Taiwan, 1993–1996. Diabet Med. 2003;20:182–185. doi: 10.1046/j.1464-5491.2003.00772.x. [DOI] [PubMed] [Google Scholar]
- 5.Tai TY, Yang CL, Chang CJ, Chang SM, Chen YH, Lin BJ, et al. Epidemiology of diabetes mellitus among adults in Taiwan, R.O.C. J Med Assoc Thai. 1987;70 Suppl 2:42–48. [PubMed] [Google Scholar]
- 6.Tsai AC, Liou JC, Chang AC. Self-reported prevalence and medication status of the major aging-associated chronic diseases in older adults in Taiwan-results of a cross-sectional national survey. Asian Journal of Health and Information Sciences. 2006;1:16–30. [Google Scholar]
- 7.Department of Health and Excutive Yuan. The prevalence of type 2 Diabetes Mellitus. [Accessed at Nov. 11, 2010];Department of Health, Taiwan. 2009 http://www.doh.gov.tw/EN2006/index_EN.aspx.
- 8.Heraclides A, Chandola T, Witte DR, Brunner EJ. Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. Diabetes Care. 2009;32:2230–2235. doi: 10.2337/dc09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 10.Asghar S, Hussain A, Ali SM, Khan AK, Magnusson A. Prevalence of depression and diabetes: a population-based study from rural Bangladesh. Diabet Med. 2007;24:872–877. doi: 10.1111/j.1464-5491.2007.02136.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 12.Knuth AG, Hallal PC. Temporal trends in physical activity: a systematic review. J Phys Act Health. 2009;6:548–559. doi: 10.1123/jpah.6.5.548. [DOI] [PubMed] [Google Scholar]
- 13.World Resources Institute. Coffee consumption. [Accessed at Nov. 11, 2010]; http://earthtrends.wri.org.
- 14.Lundsberg LS. Caffeine consumption. In: Spiller GA, editor. Caffeine. Boca Raton, FL: CRC Press; 1998. pp. 199–224. [Google Scholar]
- 15.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 16.Odegaard AO, Pereira MA, Koh WP, Arakawa K, Lee HP, Yu MC. Coffee, tea, and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2008;88:979–985. doi: 10.1093/ajcn/88.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira MA, Parker ED, Folsom AR. Coffee consumption and risk of type 2 diabetes mellitus: an 11-year prospective study of 28 812 postmenopausal women. Arch Intern Med. 2006;166:1311–1316. doi: 10.1001/archinte.166.12.1311. [DOI] [PubMed] [Google Scholar]
- 18.van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;360:1477–1478. doi: 10.1016/S0140-6736(02)11436-X. [DOI] [PubMed] [Google Scholar]
- 19.Isogawa A, Noda M, Takahashi Y, Kadowaki T, Tsugane S. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2003;361:703–704. doi: 10.1016/S0140-6736(03)12586-X. [DOI] [PubMed] [Google Scholar]
- 20.Saremi A, Tulloch-Reid M, Knowler WC. Coffee consumption and the incidence of type 2 diabetes. Diabetes Care. 2003;26:2211–2212. doi: 10.2337/diacare.26.7.2211. [DOI] [PubMed] [Google Scholar]
- 21.International Diabetes Federation. [Accessed at Nov. 11, 2010];The Diabetes Atlas, fourth edition. 2009 http://www.diabetesatlas.org/
- 22.Lin CC, Liu CS, Li TC, Chen CC, Li CI, Lin WY. Microalbuminuria and the metabolic syndrome and its components in the Chinese population. Eur J Clin Invest. 2007;37:783–790. doi: 10.1111/j.1365-2362.2007.01865.x. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Liu CS, Lai MM, Li CI, Chen CC, Chang PC, et al. Metabolic syndrome in a Taiwanese metropolitan adult population. BMC Public Health. 2007;7:239–243. doi: 10.1186/1471-2458-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin WY, Pi-Sunyer FX, Liu CS, Li TC, Li CI, Huang CY, et al. Betel nut chewing is strongly associated with general and central obesity in Chinese male middle-aged adults. Obesity (Silver Spring) 2009;17:1247–1254. doi: 10.1038/oby.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 26.Campos H, Baylin A. Coffee consumption and risk of type 2 diabetes and heart disease. Nutr Rev. 2007;65:173–179. doi: 10.1111/j.1753-4887.2007.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 27.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 28.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged U.S. women. Diabetes Care. 2006;29:398–403. doi: 10.2337/diacare.29.02.06.dc05-1512. [DOI] [PubMed] [Google Scholar]
- 29.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1999;159:2151–2159. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27:134–140. doi: 10.2337/diacare.27.1.134. [DOI] [PubMed] [Google Scholar]
- 31.Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, et al. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004;140:1–8. doi: 10.7326/0003-4819-140-1-200401060-00005. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg JA, Boozer CN, Geliebter A. Coffee, diabetes, and weight control. Am J Clin Nutr. 2006;84:682–693. doi: 10.1093/ajcn/84.4.682. [DOI] [PubMed] [Google Scholar]
- 33.van Dam RM. Coffee consumption and the decreased risk of diabetes mellitus type 2. Ned Tijdschr Geneeskd. 2006;150:1821–1825. [PubMed] [Google Scholar]
- 34.Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91:950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 35.Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554–562. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]